Prof Simon Barry
University Professorial Research Fellow (E)
School of Biomedicine
Faculty of Health and Medical Sciences
Professor Simon C BarryI have developed a collaborative research program in the discipline of Paediatrics which has both a basic science and a translational component. This allows me to undertake gene discovery and functional validation in vitro and then access human clinical material to determine whether there is a causal link with disease. I run a grant funded research group within the Robinson Research Institute, and. I currently have a group of 5 staff and 3 students working on three projects.
I have developed a collaborative research program in the discipline of Paediatrics which has both a basic science and a translational component. This allows me to undertake gene discovery and functional validation in vitro and then access human clinical material to determine whether there is a causal link with disease. I run a grant funded research group within the Robinson Research Institute, and. I currently have a group of 5 staff and 3 students working on three projects.
Basic research
My background in molecular biology and gene discovery while working at Immunex in Seattle lead me to use unbiased genome wide discovery platforms as a way to identify the key genes and pathways in a cell, and I have had a long standing interest in transcriptional regulation and how gene networks collaborate to shape a phenotype. I have applied this to the field of molecular immunology, and to the transcription factor FOXP3 in particular. FOXP3 is essential for the formation and function of regulatory T cells (Tregs), and without Treg, a fatal aggressive autoimmune disease occurs (known as IPEX in man, and SCURFY in mouse). Treg are the policemen of the immune system and are responsible for maintaining tolerance to health, and for maintaining immune homeostasis. The lack of Treg or Treg function is now accepted as part of the cause of many autoimmune diseases, including Type 1 diabetes, IBD, rheumatoid arthritis and multiple sclerosis, and hence Treg play a significant part in maintaining health. With consecutive funding from 3 NHMRC project grants, I have been able to use genome wide approaches to discover the key targets of FOXP3 in human Treg, and to validate their biological importance in vitro and in vivo. My group was the first in the world to achieve this for human FOXP3 and we discovered a key target of FOXP3, SATB1 which is regulated to maintain Treg function. This is an international collaboration, and is productivity is demonstrated by a new NHMRC grant in 2012 and a paper in Nature Immunology. I have also taken advantage of the new finding that FOXP3 may act as a tumour suppressor in human beast epithelia, and we have used our FOXP3 target discovery to uncover novel mechanisms by which FOXP3 may act as such a tumour suppressor. This project began as an honours project, grew to a Ph.D project, and received NHMRC project funding for 2012-15. We are also investigating the role of microRNAs in the regulation of the Treg gene network, and this has lead to a new collaboration on the role of miRs in reproduction and 2 NHMRC grants with Prof Sarah Robertson.
Translational Research
I also led a project team in the $68 million CRC for Biomarker Translation, which aims to discover novel Treg surface proteins with commercial partners BD and AMGEN. I bring novel surface protein candidates to this CRC from my NHMRC funded basic science project. The commercialisation process for these novel surface markers is underway, with two full US, Europe and Australia patents awarded to protect a novel cell surface biomarker of human regulatory T cells, and a cell therapy to treat diseases with a Treg defect. This project is now at the stage where we seek a commercial partner to undertake a pilot clinical trial and translate my research findings to commercial applications.
Most recently I was invited to join and lead a theme in a new CRC for cell therapy manufacturing, and this was awarded $53 million in Jan 2013. This CRC will further aid my commercialisation and translational research program by developing methods to generate a cell therapy at lower cost of goods. We have filed 2 patents from the work in this CRC, and have now launched a small Biotech start-up (Carina Biotech) to commercialise human T cell expansion technologies, and apply them for human clinical trials. This will make A Treg and CAR-T cell therapy feasible in the future, and will make it affordable for healthcare systems and for commercial applications
| Date | Position | Institution name |
|---|---|---|
| 2017 - ongoing | Professor | Robinson Research Institute |
| 2010 - ongoing | Chief Hospital Scientist | WCHN |
| 2004 - 2017 | Associate Professor | Robinson Research Insitute, University of Adelaide |
| Date | Institution name | Country | Title |
|---|---|---|---|
| 1987 - 1991 | National Institute for Medical Research | United Kingdom | Ph.D |
| 1983 - 1987 | King's College London | United Kingdom | B.Sc (hons) |
| Year | Citation |
|---|---|
| 2025 | Perveen, K., Quach, A., Stark, M. J., Barry, S. C., Prescott, S. L., & Ferrante, A. (2025). Polyunsaturated fatty acids regulate protein kinase C zeta levels in immature (cord blood) T cells, their maturation towards a Th1 cytokine bias and its significance in reducing allergy risk. In JOURNAL OF IMMUNOLOGY Vol. 214 (pp. 1 page). OXFORD UNIV PRESS. DOI |
| 2024 | Foeng, J., Tyllis, T., Abbott, C., McPeake, D., Thompson, E., Bandara, V., . . . McColl, S. (2024). Preclinical in vivo characterization underpinning LGR5-targeting CAR-T cells as a cancer immunotherapy. In CANCER RESEARCH Vol. 84 (pp. 2 pages). CA, San Diego: AMER ASSOC CANCER RESEARCH. DOI |
| 2023 | Bandara, V., Gundsambuu, B., Napoli, S., Foeng, J., McPeake, D., Tylis, T., . . . Barry, S. (2023). From bench to bedside: GMP manufacturing and testing of an LGR5-targeting CAR-T against colorectal cancer. In CANCER RESEARCH Vol. 84 (pp. 2 pages). CA, San Diego: AMER ASSOC CANCER RESEARCH. DOI WoS1 |
| 2023 | Tyllis, T., Abbott, C., McPeake, D., Foeng, J., Bandara, V., Gundsambuu, B., . . . McColl, S. (2023). Development of a flow cytometry-based assay for measuring specific CAR expression on LGR5-targeting CAR-T cells. In CANCER RESEARCH Vol. 83 (pp. 2 pages). Orlando, FL: AMER ASSOC CANCER RESEARCH. DOI |
| 2023 | Bandara, V., Gundsambuu, B., Napoli, S., Mills, S., Thompson, E., Tan, L., . . . Barry, S. C. (2023). Development and in vitro validation of an LGR-5 targeting CAR-T against colorectal cancer. In CANCER RESEARCH Vol. 83 (pp. 2 pages). Orlando, FL: AMER ASSOC CANCER RESEARCH. DOI WoS1 |
| 2023 | McPeake, D., Tyllis, T., Abbott, C., Foeng, J., Bandara, V., Gundsambuu, B., . . . McColl, S. (2023). In vivo efficacy of LGR5-targeting CAR-T cell therapies developed for the treatment of colorectal cancer. In CANCER RESEARCH Vol. 83 (pp. 2 pages). Orlando, FL: AMER ASSOC CANCER RESEARCH. DOI |
| 2023 | Thompson, E. J., Bandara, V., Gundsambuu, B., Napoli, S., Mills, S., Foeng, J., . . . Bonder, C. S. (2023). Assessing LGR5 expression levels on colorectal cancer tissue samples for use in LGR5-targeting CAR-T cell therapy clinical trial. In CANCER RESEARCH Vol. 83 (pp. 2 pages). Orlando, FL: AMER ASSOC CANCER RESEARCH. DOI WoS1 |
| 2023 | Desai, J., Iglesias, J. L., Jablonskis, L. T., McColl, S., Barry, S. C., & Morelli, M. P. (2023). A phase 1/2a, multicenter, open-label study of CNA3103 (LGR5-targeted, autologous CART cells) in patients with metastatic colorectal cancer (mCRC). In JOURNAL OF CLINICAL ONCOLOGY Vol. 41 (pp. 1 page). IL, Chicago: LIPPINCOTT WILLIAMS & WILKINS. WoS3 |
| 2022 | Rad, E. R., Foeng, J., McPeak, D., Tyllis, T., Abbott, C., Bandara, V., . . . McColl, S. (2022). ADAM10-targeting CAR-T cells inhibit colon cancer cell growth <i>in vivo</i>. In CANCER RESEARCH Vol. 82 (pp. 2 pages). LA, New Orleans: AMER ASSOC CANCER RESEARCH. |
| 2022 | Wang, W., Bandara, V., Lokman, N., Napoli, S., Gundsambuu, B., Oehler, M., . . . Ricciardelli, C. (2022). LGR5 CAR-T cells: A novel potential treatment against high grade serous ovarian cancer.. In CANCER RESEARCH Vol. 82 (pp. 2 pages). LA, New Orleans: AMER ASSOC CANCER RESEARCH. |
| 2022 | Bandara, V., Mills, S., Thompson, E., Tan, L., Gundsambuu, B., Napoli, S., . . . Barry, S. (2022). Pre-clinical validation of a LGR5-targeting CAR-T against colorectal cancer.. In CANCER RESEARCH Vol. 82 (pp. 1 page). LA, New Orleans: AMER ASSOC CANCER RESEARCH. |
| 2022 | Thompson, E. J., Bandara, V., Sadlon, T., Gundsambuu, B., Tan, L. Y., Ricciardelli, C., . . . Bonder, C. S. (2022). Real-time cytotoxicity assays as a pre-clinical screening tool for LGR5-targeting CAR-T cells for treatment of solid tumors. In CANCER RESEARCH Vol. 82 (pp. 1 page). LA, New Orleans: AMER ASSOC CANCER RESEARCH. |
| 2022 | Thompson, E. J., Bandara, V., Sadlon, T., Gundsambuu, B., Tan, L. Y., Ricciardelli, C., . . . Bonder, C. S. (2022). Real-time cytotoxicity assays as a pre-clinical screening tool for LGR5-targeting CAR-T cells for treatment of solid tumors.. In CANCER RESEARCH Vol. 82 (pp. 1 page). LA, New Orleans: AMER ASSOC CANCER RESEARCH. |
| 2022 | McPeake, D. J., Tyllis, T. S., Foeng, J., Bandara, V., Abbott, C. A., Gundsambuu, B., . . . McColl, S. R. (2022). <i>In vivo</i> characterization of a novel CAR-T cell therapy directed towards LGR5 for the treatment of colorectal cancer.. In CANCER RESEARCH Vol. 82 (pp. 2 pages). LA, New Orleans: AMER ASSOC CANCER RESEARCH. |
| 2022 | McPeake, D. J., Tyllis, T. S., Foeng, J., Bandara, V., Abbott, C. A., Gundsambuu, B., . . . McColl, S. R. (2022). In <i>vivo</i> characterization of a novel CAR-T cell therapy directed towards LGR5 for the treatment of colorectal cancer. In CANCER RESEARCH Vol. 82 (pp. 2 pages). LA, New Orleans: AMER ASSOC CANCER RESEARCH. |
| 2022 | Bandara, V., Mills, S., Thompson, E., Tan, L., Gundsambuu, B., Napoli, S., . . . Barry, S. (2022). Pre-clinical validation of a LGR5-targeting CAR-T against colorectal cancer. In CANCER RESEARCH Vol. 82 (pp. 1 page). LA, New Orleans: AMER ASSOC CANCER RESEARCH. |
| 2022 | Wang, W., Bandara, V., Lokman, N., Napoli, S., Gundsambuu, B., Oehler, M., . . . Ricciardelli, C. (2022). LGR5 CAR-T cells: A novel potential treatment against high grade serous ovarian cancer. In CANCER RESEARCH Vol. 82 (pp. 2 pages). LA, New Orleans: AMER ASSOC CANCER RESEARCH. |
| 2022 | Rad, E. R., Foeng, J., McPeak, D., Tyllis, T., Abbott, C., bandara, V., . . . McColl, S. (2022). ADAM10-targeting CAR-T cells inhibit colon cancer cell growth <i>in vivo</i>.. In CANCER RESEARCH Vol. 82 (pp. 2 pages). LA, New Orleans: AMER ASSOC CANCER RESEARCH. |
| 2022 | Tunbridge, M. J., Perkins, G., Salehi, T., Grubor-Bauk, B., Sim, B., Ying, T., . . . Coates, P. T. (2022). A Prospective Randomised, Controlled Trial Switching Sirolimus for Mycophenolate to Enhance Immunological Responses to Third Dose COVID-19 Vaccination in Kidney Transplant Recipients with Poor Baseline Humoral Immunity. In AMERICAN JOURNAL OF TRANSPLANTATION Vol. 22 (pp. 1095). ELECTR NETWORK: WILEY. |
| 2022 | Perkins, G. B., Tunbridge, M., Salehi, T., Chai, C., Hope, C. M., Garcia-Valtanen, P., . . . Coates, P. (2022). Sirolimus Use is Associated with an Improved Immune Response to COVID-19 Vaccination in Kidney Transplant Recipients. In AMERICAN JOURNAL OF TRANSPLANTATION Vol. 22 (pp. 873). WILEY. |
| 2022 | Perkins, G., Tunbridge, M., Salehi, T., Chai, C. S., Hope, C. M., Garcia-Valtanen, P., . . . Coates, A. O. T. (2022). MTOR Inhibitors Promote Highly Functional T Cell Immunity in Kidney Transplant Recipients Vaccinated Against COVID-19. In TRANSPLANTATION Vol. 106 (pp. S223). ELECTR NETWORK: LIPPINCOTT WILLIAMS & WILKINS. |
| 2020 | Kim, J., Hope, C. M., Scaffidi, J. C., Stead, S. O., Perkins, G. B., Kette, F. D., . . . Coates, P. T. (2020). GENERATION OF STABLE HUMAN INDUCED REGULATORY T-CELLS REQUIRES OPTIMAL RAPAMYCIN CONCENTRATION AND TCR STIMULATION. In TRANSPLANTATION Vol. 104 (pp. S206). ELECTR NETWORK: LIPPINCOTT WILLIAMS & WILKINS. |
| 2018 | Bird, T. G., Mueller, M., Boulter, L., Vincent, D., Ridgway, R., Lopez Guadamillas, E., . . . Forbes, S. J. (2018). Inhibiting Senescence Induced By Macrophage Derived Tgf beta Restores a Regenerative Response and Improves Survival Following Acetaminophen Poisoning. In HEPATOLOGY Vol. 68 (pp. 200A-201A). San Francisco, CA: WILEY. |
| 2018 | Harbison, J. E., Roth-Schulze, A. J., Barry, S. C., Tran, C. D., Ngui, K., Penno, M. A., . . . Couper, J. (2018). Gut Microbiome Dysbiosis and Increased Intestinal Permeability in Australian Children with Islet Autoimmunity and Type 1 Diabetes. In DIABETES Vol. 67 (pp. 2 pages). Orlando, FL: AMER DIABETES ASSOC. DOI WoS2 |
| 2018 | Robertson, S. A., Zhang, B., Barry, S. C., Schjenken, J. E., & Moldenhauer, L. M. (2018). miRNA-155 is required to induce competent regulatory T cells and to protect against inflammation-induced fetal loss in mice. In CLINICAL ENDOCRINOLOGY Vol. 89 (pp. 36-37). Perth, AUSTRALIA: WILEY. |
| 2018 | Kim, J., Yue, Z., Liu, X., Hope, C., Rojas-Canales, D., Drogemuller, C., . . . Coates, P. T. (2018). Characterization of 3D-Printed Human Regulatory T-Cells. In TRANSPLANTATION Vol. 102 (pp. S109). Madrid, SPAIN: LIPPINCOTT WILLIAMS & WILKINS. DOI |
| 2017 | Kim, K. W., Allen, D. W., Briese, T., Pang, C. N., Jain, K., Horton, J. L., . . . Craig, M. E. (2017). Gut Virome Dynamics during Pregnancy in Mothers with Type 1 Diabetes. In Abstracts of the 77th Scientific Sessions of the American Diabetes Association, as published in Diabetes Vol. 66 (pp. LB192). San Diego, CA: American Diabetes Association. |
| 2016 | Mohandas, A., Hill, D., Hope, C., Pederson, S., Walsh, J., Grose, R., . . . Barry, S. C. (2016). A new biomarker of stable regulatory T cell function which has diagnostic utility in type 1 diabetes. In EUROPEAN JOURNAL OF IMMUNOLOGY Vol. 46 (pp. 748). Melbourne, AUSTRALIA: WILEY-BLACKWELL. |
| 2016 | Malatesta, K., Sadlon, T., Pederson, S., Brown, C., & Barry, S. C. (2016). Validating the contribution of microRNAs and lncRNAs to the molecular signature and plasticity of iTreg vs. nTreg in humans. In EUROPEAN JOURNAL OF IMMUNOLOGY Vol. 46 (pp. 1216). Melbourne, AUSTRALIA: WILEY-BLACKWELL. |
| 2016 | Brown, C., Beyer, M., Pederson, S., Goodall, G., Schultze, J., Sadlon, T., & Barry, S. C. (2016). FOXP3 tightly controls target gene expression using microRNAs in human regulatory T cells, and regulates Treg plasticity. In EUROPEAN JOURNAL OF IMMUNOLOGY Vol. 46 (pp. 1216). Melbourne, AUSTRALIA: WILEY-BLACKWELL. |
| 2016 | Harding, F., Delalat, B., Guundsambuu, B., Pardo, E., Hutmacher, D., Voelcker, N., & Barry, S. C. (2016). A novel human T cell expansion technology for affordable cell therapy. In EUROPEAN JOURNAL OF IMMUNOLOGY Vol. 46 (pp. 747). Melbourne, AUSTRALIA: WILEY-BLACKWELL. |
| 2015 | Muhling, J., Russell, D., & Barry, S. (2015). USE OF VIRAL VECTORS IN PRIMARY CELLS. In JOURNAL OF GENE MEDICINE Vol. 17 (pp. 214-215). Univ Melbourne, Univ Coll, Parkville, AUSTRALIA: WILEY-BLACKWELL. |
| 2014 | Mohandas, A., Zola, H., Barry, S., & Krumbiegel, D. (2014). Peptidase inhibitor 16 identifies a unique subset of memory T helper cells with hyperproliferative and proinflammatory properties. In JOURNAL OF IMMUNOLOGY Vol. 192 (pp. 1 page). AMER ASSOC IMMUNOLOGISTS. |
| Year | Citation |
|---|---|
| 2014 | Barry, S. (2014). 2408934, Peptidase Inhibitor 16(PI16) as a Biomarker for Regulatory T (TREG) Cells and Uses Thereof. Europe. |
| Year | Citation |
|---|---|
| 2018 | Pederson, S. M. (2018). BMEA: Bayesian Modelling for Exon Array Data. (PhD Thesis, University of Adelaide). |
| Year | Citation |
|---|---|
| 2021 | Ryan, F., Hope, C., Masavuli, M., Lynn, M., Mekonnen, Z., Lip Yeow, A. E., . . . Lynn, D. (2021). Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection. DOI Europe PMC1 |
Summary of major funding
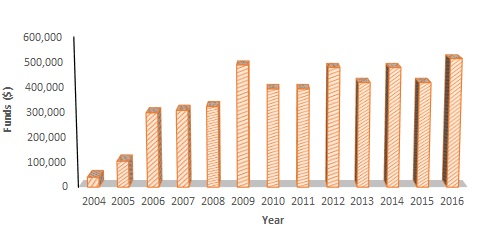
Since my arrival in Jan 2004, I have been successful in attracting over $3.2 million in research funding ($1,210,500 currently to my lab), including 5 NHMRC project grants as CIA, an NHMRC program grant as CID and an ARC linkage grant as CIA. As a co senior applicant I am a CI on 2 NHMRC project grants, and 2 Cooperative Research Centre programs. In collaboration with a national team lead by Prof Couper I am a named chief investigator on the ENDIA study, a $10 million birth cohort study funded by the JDRF and Helmsley trust. In addition, I have attracted a number of small grants as CIA and a commercial partnership. I am named CI on 4 NHMRC project applications under consideration in the current round. From 2006, my successful grant application rate is over 80%. This has allowed growth in staff in my group, and allowed a number of collaborations to be initiated, and these are now yielding high impact publications.
Teaching
Annual lecture delivery to year 3 Infection and Immunity course in the Bachelor of Science degree (Faculty of Science, School of Biological Sciences, Department of Molecular and Cellular Biology. This course is coordinated by Prof Shaun McColl, and is managed by Chris Wong and Jamie Botten. I have been delivering a research and training lecture to this course since 2006, and although I have not received SELT scores for my contribution to this, I have been invited to teach in this course for 8 years, and I have doubled the number of lectures I deliver since 2014.
| Date | Role | Research Topic | Program | Degree Type | Student Load | Student Name |
|---|---|---|---|---|---|---|
| 2023 | Co-Supervisor | Investigating the role of JAZF1 in the formation and function of human induced Regulatory T cells | Doctor of Philosophy | Doctorate | Full Time | Miss Hannah Rae Morgan |
| 2023 | Principal Supervisor | Investigating regulatory T cells post COVID-19 | Doctor of Philosophy | Doctorate | Full Time | Ms Sarah May Battersby |
| 2023 | Principal Supervisor | Investigating regulatory T cells post COVID-19 | Doctor of Philosophy | Doctorate | Full Time | Ms Sarah May Battersby |
| 2023 | Co-Supervisor | Investigating the role of JAZF1 in the formation and function of human induced Regulatory T cells | Doctor of Philosophy | Doctorate | Full Time | Miss Hannah Rae Morgan |
| 2022 | Principal Supervisor | Optimisation of Chimeric Antigen Receptor T Cells for Pan Solid Tumour Immunotherapy | Doctor of Philosophy | Doctorate | Full Time | Mr Jieren Zheng |
| 2022 | Principal Supervisor | Optimisation of Chimeric Antigen Receptor T Cells for Pan Solid Tumour Immunotherapy | Doctor of Philosophy | Doctorate | Full Time | Mr Jieren Zheng |
| Date | Role | Research Topic | Program | Degree Type | Student Load | Student Name |
|---|---|---|---|---|---|---|
| 2020 - 2024 | Co-Supervisor | Development of novel ovarian cancer treatment using CAR-T cells targeting LGR5 | Doctor of Philosophy | Doctorate | Full Time | Ms Wanqi Wang |
| 2018 - 2020 | Co-Supervisor | Three-Dimensional Printing of Regulatory T-Cells: A Novel Immunotherapy to Prevent Transplantation Rejection | Doctor of Philosophy | Doctorate | Full Time | Mr Juewan Kim |
| 2017 - 2021 | Co-Supervisor | Three-dimensional regulation: Establishing novel linkages between non-coding genetic variation and target genes | Doctor of Philosophy | Doctorate | Full Time | Mr Ning Liu |
| 2017 - 2021 | Principal Supervisor | The Identification Of Genetic And Epigenetic Changes That Contribute To Type 1 Diabetes | Doctor of Philosophy | Doctorate | Full Time | Dr Ying Ying Wong |
| 2016 - 2021 | Principal Supervisor | A NOVEL ROLE FOR ZEB2 AS A LINEAGE FIDELITY CHECKPOINT IN HUMAN CD4+ T CELLS | Doctor of Philosophy | Doctorate | Full Time | Mr Soon Wei Wong |
| 2016 - 2023 | Co-Supervisor | Protein Kinase C zeta Regulates the Development of Cord Blood T Cells from the Immature Th2 to the Mature Th1 Cytokine Phenotype and its Implication in Development of Allergic Sensitization | Doctor of Philosophy | Doctorate | Full Time | Dr Khalida Perveen |
| 2015 - 2018 | Co-Supervisor | Hormone and transcription factor regulation of cytokines in the mammary gland | Doctor of Philosophy | Doctorate | Full Time | Mr Vahid Atashgaran |
| 2013 - 2021 | Co-Supervisor | DOES THE GUT MICROBIOME DRIVE THE DEVELOPMENT OF TYPE 1 DIABETES? Type 1 diabetes and the gut (TIGs) cohort | Doctor of Philosophy | Doctorate | Part Time | Dr Jessica Emily Harbison |
| 2013 - 2018 | Co-Supervisor | T Regulatory Cells in Early Pregnancy in Mice | Doctor of Philosophy | Doctorate | Full Time | Miss Bihong Zhang |
| 2011 - 2013 | Co-Supervisor | Longevity of Airway Gene Therapy for Cystic Fibrosis: Single and Repeat Lentiviral Dosing | Doctor of Philosophy | Doctorate | Part Time | Dr Patricia Lucia Cmielewski |
| 2011 - 2015 | Co-Supervisor | Characterisation of PI16+ T helper cells | Doctor of Philosophy | Doctorate | Full Time | Mr Arunesh Pullaniparambil Mohandas |
| 2011 - 2015 | Co-Supervisor | Cystic Fibrosis: The role of airway stem cells in sustained gene expression by lentiviral directed gene therapy | Doctor of Philosophy | Doctorate | Full Time | Dr Nigel Farrow |
| 2009 - 2013 | Principal Supervisor | A Tumour Suppressor Role for FOXP3 and FOXP3-regulated MicroRNAs in Breast Cancer Cells | Doctor of Philosophy | Doctorate | Full Time | Miss Natasha McInnes |
| 2008 - 2018 | Principal Supervisor | BMEA: Bayesian Modelling for Exon Array Data | Doctor of Philosophy | Doctorate | Part Time | Dr Stephen Martin Pederson |
| 2006 - 2009 | Co-Supervisor | Regulatory T Cells, Th17 Effector Cells and Cytokine Microenvironment in Inflammatory Bowel Disease and Coeliac Disease | Doctor of Philosophy | Doctorate | Part Time | Dr Nicola Eastaff-Leung |
| Date | Role | Board name | Institution name | Country |
|---|---|---|---|---|
| 2015 - ongoing | Board Member | Cell bank Australia | children's medical research institute | Australia |
| Date | Role | Committee | Institution | Country |
|---|---|---|---|---|
| 2014 - ongoing | Member | RRI Executive | Robinson Research Institute | Australia |
| 2009 - ongoing | Vice-Chair | WCHN IBC | WCHN | Australia |
| Date | Role | Editorial Board Name | Institution | Country |
|---|---|---|---|---|
| 2016 - ongoing | Associate Editor | Frontiers in Immunology | Robinson Research Insitute | Australia |
| Date | Engagement Type | Partner Name |
|---|---|---|
| 2011 - ongoing | Consultant | BeckTon Dickenson |






