
APrf Timothy Sargeant
Internal Grant-Funded Researcher D
School of Pharmacy and Biomedical Sciences
College of Health
Eligible to supervise Masters and PhD - email supervisor to discuss availability.
A/Prof Tim Sargeant. BBMedSc. (Human Genetics), Hons. 1st Class, PhD, Victoria University of Wellington (New Zealand). Please find my socials in the contact tab below, or email me at SAHMRI: Tim.Sargeant@SAHMRI.com. Addressing the Lifespan-Healthspan Gap: while the 20th century brought remarkable increases in lifespan, reducing the gap between lifespan and healthspan remains a significant challenge. In countries like Australia, the last decade of life is often dominated by age-related diseases. To close this gap, we must address the fundamental drivers of cellular ageing. Autophagy and lysosomal activity are vital cellular processes that recycle damaged and unwanted material. These mechanisms are crucial for maintaining cellular health during ageing. When they are impaired, biological ageing accelerates, contributing to age-related diseases like atherosclerosis and dementia — leading causes of death in Australia. A/Prof Tim Sargeant’s team has developed innovative tools to measure autophagy and lysosomal activity in humans and is working on therapies to enhance this recycling machinery. By targeting the biology of ageing itself, their research seeks to improve healthspan and provide transformative solutions to combat age-related diseases at their root. Scientific Background and Research Focus: A/Prof Tim Sargeant began his scientific journey with a PhD in neuroscience at Victoria University of Wellington, New Zealand. He subsequently undertook two postdoctoral fellowships at the University of Cambridge (UK), where he gained expertise in molecular and cell biology, focusing on the lysosomal system—the cell’s recycling machinery. In 2015, Tim was appointed Head of Lysosomal Health in Ageing at the South Australian Health and Medical Research Institute (SAHMRI). His research investigates the role of the lysosomal system in biological ageing, with an emphasis on its ability to clear damaged and unwanted cellular material. By understanding how lysosomal recycling slows cellular ageing, Tim’s work addresses how this process underpins the prevention and treatment of age-related diseases.
Research Overview
Preclinical studies have established that autophagy is vital for healthy ageing, with enhanced autophagy promoting longer healthspans in disease models. However, our understanding of autophagy in humans remains limited. This gap presents a significant challenge for drug development, as pharmaceutical companies cannot readily measure autophagy activity in clinical trials, thereby hindering the translation of autophagy-enhancing drugs and interventions.
To address this critical issue, our research has focused on developing innovative approaches to measure autophagy and lysosomal function in humans. We currently have three active projects:
- Direct measurement of autophagy using human blood.
- Development of biomarkers for autophagy in human biofluids.
- Imaging lysosomal function using positron emission tomography (PET).
This research program has been supported by including but not limited to three NHMRC Ideas Grants and a BrightFocus grant (A/Prof Sargeant as lead investigator).
Direct Measurement of Autophagy Using Human Blood
Overview
The direct measurement of autophagic flux in whole human blood represents our most advanced autophagy assessment technology. This method captures critical data about autophagy within a physiologically intact environment, an essential factor as autophagy is highly sensitive to disruptions.
Methodology
Autophagic flux is measured by detecting LC3B-II through techniques such as western blot, flow cytometry, or ELISA, as illustrated in the accompanying figure.
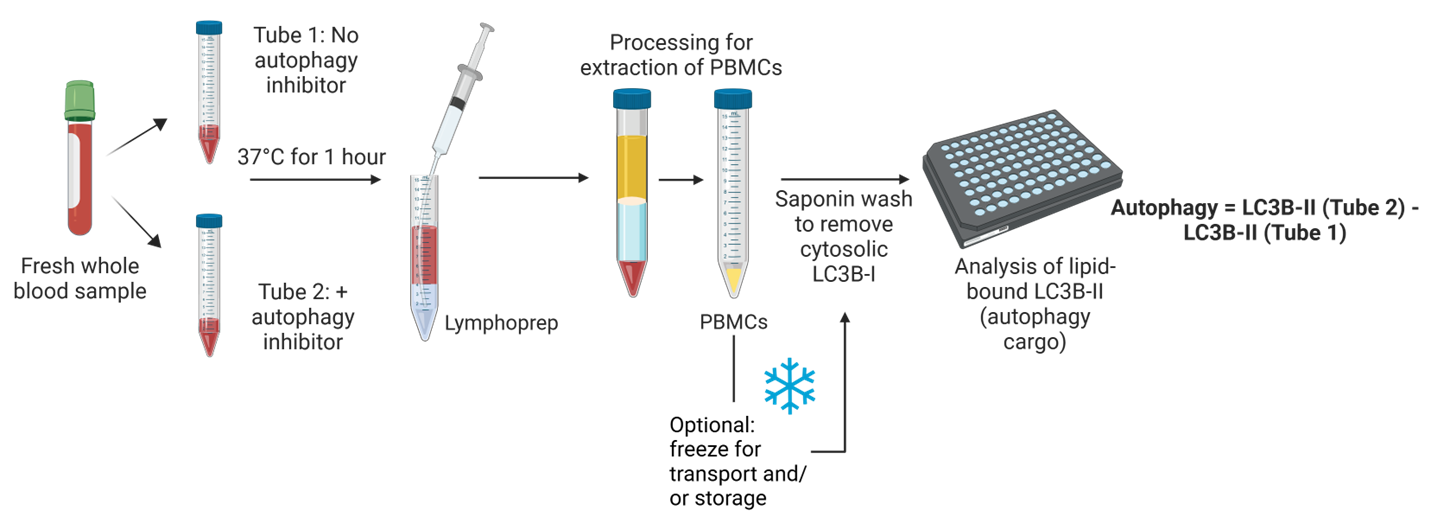
Milestones and Applications
Since its initial publication in 2021, this method has been adopted in studies by our research group and external collaborators.
Key Publications (please find a full list of publications here - A/Prof TJ Sargeant full publication list)
- Research from Lysosomal Health in Ageing (SAHMRI) - measurement of autophagy in humans:
- Dang et al Publication
- Bensalem et al Publication
- Singh et al Publication
- Bensalem et al Publication
- Research from Lysosomal Health in Ageing (SAHMRI) - autophagy removes aggregated tau in human neurons:
- Carosi et al Publication
- Collaborative research with Lysosomal Health in Ageing (SAHMRI):
- Masedunskas et al Publication
- Find our latest perspectives on autophagy in this Nature Cell Biology Comment article:
- Singh et al Publication
- Research from external groups:
- Mackert et al Publication
- Cooper et al Preprint
Intellectual Property
This technology is protected by patent in the UK (GB2603664B), with patents pending in Australia and the United States.
Development of Biomarkers for Autophagy in Human Biofluids
Addressing a Critical Gap
Our autophagy biomarker programme focuses on discovering and validating blood-borne molecules indicative of intracellular autophagy. While not as mature as our direct autophagy blood test, this initiative is vital for advancing the field.
Strengths and Capabilities
- Mechanistic Insights: Our team of world-class cell biologists investigates the mechanistic links between candidate biomarkers and autophagy, ensuring a deep understanding beyond simple correlations.
- Integrated Approach: Collaborations with SAHMRI's Bioresources and Clinical Trials Platform enable us to validate candidate biomarkers in both preclinical models and human studies.
Impact
Validated biomarkers will enable clinic- or home-friendly autophagy measurements, a key step in translating fundamental ageing science into practical applications.
Imaging Lysosomal Function Using Positron Emission Tomography (PET)
Expanding the Toolkit
While biofluid-based tests are crucial for assessing autophagy, imaging the lysosomal system directly within tissues offers unique insights. This programme aims to develop PET imaging technologies to predict disease emergence, such as the hallmarks of Alzheimer’s disease in the brain.
Current Status
This initiative is in its early stages and is being conducted in collaboration with the Molecular Imaging and Therapy Research Unit (MITRU) at SAHMRI.
Future Vision
Advanced imaging technologies will provide a transformative approach to understanding lysosomal health and predicting disease at the tissue level.
| Date | Position | Institution name |
|---|---|---|
| 2024 - ongoing | Associate Professor (Affiliate) | University of Adelaide |
| Date | Institution name | Country | Title |
|---|---|---|---|
| Victoria University of Wellington | New Zealand | PhD |
| Year | Citation |
|---|---|
| 2026 | Hein, L. K., Hattersley, K. J., Bensalem, J., & Sargeant, T. J. (2026). Measurement of Physiological Autophagic Flux in the Human Peripheral Blood Mononuclear Cell Pool. Methods in molecular biology (Clifton, N.J.), 2976, 61-72. |
| 2025 | Okugbeni, N., Cole, V., Kroon, E., Sargeant, T. J., Loos, B., & Kinnear, C. (2025). Harnessing patient autophagy flux to transform tuberculosis treatment. Trends in Molecular Medicine, S1471-4914(25)00287-4. |
| 2025 | Singh, S., Carosi, J. M., Dang, L., & Sargeant, T. J. (2025). Autophagy does not always decline with ageing. Nature Cell Biology, 27(5), 712-715. Scopus2 WoS2 Europe PMC2 |
| 2025 | Bensalem, J., Teong, X. T., Hattersley, K. J., Hein, L. K., Fourrier, C., Dang, L. V. P., . . . Sargeant, T. J. (2025). Intermittent time-restricted eating may increase autophagic flux in humans: an exploratory analysis.. Journal of Physiology, 603(10), 3019-3032. Scopus5 WoS4 Europe PMC4 |
| 2025 | Carosi, J. M., Martin, A., Hein, L. K., Hassiotis, S., Hattersley, K. J., Turner, B. J., . . . Sargeant, T. J. (2025). Autophagy across tissues of aging mice. PLoS ONE, 20(6), e0325505-1-e0325505-17. Scopus2 WoS1 Europe PMC3 |
| 2025 | Dang, L. V. P., Martin, A., Carosi, J. M., Gore, J., Singh, S., & Sargeant, T. J. (2025). Cell-Type-Specific Autophagy in Human Leukocytes. The FASEB Journal, 39(12), e70708-1-e70708-13. Scopus4 WoS2 Europe PMC2 |
| 2025 | Singh, S., Fourrier, C., Hattersley, K. J., Hein, L. K., Gore, J., Martin, A., . . . Sargeant, T. J. (2025). High protein does not change autophagy in human peripheral blood mononuclear cells after one hour. JCI Insight, 10(16), e188845-1-e188845-3. |
| 2025 | Dang, L. V., & Sargeant, T. J. (2025). Cell type-specific autophagy in human leukocytes: signatures of aging, sex, and nutrient restriction.. Autophagy Rep, 4(1), 2543560. Scopus1 Europe PMC1 |
| 2024 | Carosi, J. M., Hein, L. K., Sandow, J. J., Dang, L. V. P., Hattersley, K., Denton, D., . . . Sargeant, T. J. (2024). Autophagy captures the retromer-TBC1D5 complex to inhibit receptor recycling. Autophagy, 20(4), 863-882. Scopus8 WoS8 Europe PMC10 |
| 2024 | Singh, S., Bensalem, J., Hein, L. K., Casey, A., Mäkinen, V. -P., & Sargeant, T. J. (2024). epHero - a tandem-fluorescent probe to track the fate of apoptotic cells during efferocytosis.. Cell Death Discov, 10(1), 179. WoS2 Europe PMC1 |
| 2024 | Lloyd-Lewis, B., D’Angelo, M. E., Prowting, N. B., Wiseman, B. E., Sargeant, T. J., & Watson, C. J. (2024). Methods for investigating STAT3 regulation of lysosomal function in mammary epithelial cells. Journal of Mammary Gland Biology and Neoplasia, 29(1), 15 pages. Scopus1 WoS1 Europe PMC1 |
| 2024 | Masedunskas, A., de Ciutiis, I., Hein, L. K., Ge, A., Kong, Y. X., Qi, M., . . . Fontana, L. (2024). Investigating the Impact of Glycogen-Depleting Exercise Combined with Prolonged Fasting on Autophagy and Cellular Health in Humans: A Randomised Controlled Crossover Trial. Nutrients, 16(24), 4297-1-4297-13. Scopus2 WoS2 Europe PMC1 |
| 2023 | Carosi, J. M., & Sargeant, T. J. (2023). Rapamycin and Alzheimer disease: a hypothesis for the effective use of rapamycin for treatment of neurodegenerative disease.. Autophagy, 19(8), 1-5. Scopus28 WoS28 Europe PMC28 |
| 2023 | Bensalem, J., Hein, L. K., Hassiotis, S., Trim, P. J., Proud, C. G., Heilbronn, L. K., & Sargeant, T. J. (2023). Modifying dietary protein impacts mTOR signaling and brain deposition of amyloid beta in a knock-in mouse model of Alzheimer's disease.. Journal of Nutrition, 153(5), 1407-1419. Scopus4 WoS4 Europe PMC4 |
| 2023 | Schwarz, N., Fernando, S., Chen, Y. -C., Salagaras, T., Rao, S. R., Liyanage, S., . . . Psaltis, P. J. (2023). Colchicine exerts anti-atherosclerotic and -plaque-stabilizing effects targeting foam cell formation. The FASEB Journal, 37(4), 1-20. Scopus59 WoS58 Europe PMC49 |
| 2023 | Teong, X. T., Liu, K., Vincent, A. D., Bensalem, J., Liu, B., Hattersley, K. J., . . . Heilbronn, L. K. (2023). Intermittent fasting plus early time-restricted eating versus calorie restriction and standard care in adults at risk of type 2 diabetes: a randomized controlled trial. Nature Medicine, 29(4), 963-972. Scopus95 WoS86 Europe PMC73 |
| 2023 | Carosi, J. M., Denton, D., Kumar, S., & Sargeant, T. J. (2023). Receptor Recycling by Retromer. Molecular and Cellular Biology, 43(7), 317-334. Scopus18 WoS18 Europe PMC16 |
| 2023 | Bensalem, J., Teong, X. T., Hattersley, K. J., Hein, L. K., Fourrier, C., Liu, K., . . . Sargeant, T. J. (2023). Basal autophagic flux measured in blood correlates positively with age in adults at increased risk of type 2 diabetes. Geroscience, 45(6), 3549-3560. Scopus15 WoS13 Europe PMC12 |
| 2023 | Sargeant, T. J., & Fourrier, C. (2023). Human monocyte-derived microglia-like cell models: A review of the benefits, limitations and recommendations. Brain Behavior and Immunity, 107, 98-109. Scopus19 WoS17 Europe PMC19 |
| 2022 | Bensalem, J., Heilbronn, L. K., Gore, J. R., Hutchison, A. T., Sargeant, T. J., & Fourrier, C. (2022). The Break-Fast study protocol: a single arm pre-post study to measure the effect of a protein-rich breakfast on autophagic flux in fasting healthy individuals. BMC Nutrition, 8(1), 120-1-120-7. Scopus1 WoS1 Europe PMC1 |
| 2022 | Casey, A. E., Liu, W., Hein, L. K., Sargeant, T. J., Pederson, S. M., & Mäkinen, V. P. (2022). Transcriptional targets of senataxin and E2 promoter binding factors are associated with neuro-degenerative pathways during increased autophagic flux. Scientific Reports, 12(1, article no. 17665), 1-12. Scopus2 WoS2 Europe PMC2 |
| 2022 | Carosi, J. M., Fourrier, C., Bensalem, J., & Sargeant, T. J. (2022). The mTOR–lysosome axis at the centre of ageing. FEBS Open Bio, 12(4), 739-757. Scopus51 WoS48 Europe PMC43 |
| 2022 | Whyte, L. S., Fourrier, C., Hassiotis, S., Lau, A. A., Trim, P. J., Hein, L. K., . . . Sargeant, T. J. (2022). Lysosomal gene Hexb displays haploinsufficiency in a knock-in mouse model of Alzheimer's disease. IBRO Neuroscience Reports, 12, 131-141. Scopus11 WoS11 Europe PMC13 |
| 2022 | Casey, A., Liu, W., Hein, L., Sargeant, T., Pederson, S., & Mäkinen, V. -P. (2022). Transcriptional targets of senataxin and E2 promoter binding factors are associated with neuro-degenerative pathways during increased autophagic flux. |
| 2022 | Chaudhary, R., Liu, B., Bensalem, J., Sargeant, T. J., Page, A. J., Wittert, G. A., . . . Heilbronn, L. K. (2022). Intermittent fasting activates markers of autophagy in mouse liver, but not muscle from mouse or humans. Nutrition, 101, 1-7. Scopus22 WoS20 Europe PMC22 |
| 2022 | Vidanapathirana, A. K., Goyne, J. M., Williamson, A. E., Pullen, B. J., Chhay, P., Sandeman, L., . . . Bursill, C. A. (2022). Biological Sensing of Nitric Oxide in Macrophages and Atherosclerosis Using a Ruthenium-Based Sensor. Biomedicines, 10(8), 1807. Scopus8 WoS7 Europe PMC6 |
| 2021 | Shoubridge, A. P., Fourrier, C., Choo, J. M., Proud, C. G., Sargeant, T. J., & Rogers, G. B. (2021). Gut Microbiome Regulation of Autophagic Flux and Neurodegenerative Disease Risks. Frontiers in Microbiology, 12, 10 pages. Scopus10 WoS12 Europe PMC11 |
| 2021 | Carosi, J. M., Denton, D., Kumar, S., & Sargeant, T. J. (2021). Retromer dysfunction at the nexus of tauopathies. Cell Death and Differentiation, 28(3), 884-889. Scopus15 WoS13 Europe PMC17 |
| 2021 | Klionsky, D. J., Abdel Aziz, A. K., Abdelfatah, S., Abdellatif, M., Kumar, S., & Tong, C. K. (2021). Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy, 17(1), 1-382. Scopus2090 WoS1965 Europe PMC1991 |
| 2021 | Carosi, J. M., Hein, L. K., van den Hurk, M., Adams, R., Milky, B., Singh, S., . . . Sargeant, T. J. (2021). Retromer regulates the lysosomal clearance of MAPT/tau. Autophagy, 17(9), 2217-2237. Scopus30 WoS34 Europe PMC35 |
| 2021 | Xie, J., De Poi, S. P., Humphrey, S. J., Hein, L. K., Bruning, J. B., Pan, W., . . . Proud, C. G. (2021). TSC-insensitive Rheb mutations induce oncogenic transformation through a combination of constitutively active mTORC1 signalling and proteome remodelling. Cellular and Molecular Life Sciences, 78(8), 4035-4052. Scopus6 WoS6 Europe PMC5 |
| 2021 | Bensalem, J., Fourrier, C., Hein, L. K., Hassiotis, S., Proud, C. G., & Sargeant, T. J. (2021). Inhibiting mTOR activity using AZD2014 increases autophagy in the mouse cerebral cortex. Neuropharmacology, 190, 108541. Scopus11 WoS11 Europe PMC12 |
| 2021 | Hattersley, K. J., Carosi, J. M., Hein, L. K., Bensalem, J., & Sargeant, T. J. (2021). PICALM regulates cathepsin D processing and lysosomal function.. Biochemical and biophysical research communications, 570, 103-109. Scopus8 WoS8 Europe PMC10 |
| 2021 | Lynn, M. A., Eden, G., Ryan, F. J., Bensalem, J., Wang, X., Blake, S. J., . . . Lynn, D. J. (2021). The composition of the gut microbiota following early-life antibiotic exposure affects host health and longevity in later life. Cell Reports, 36(8), 20 pages. Scopus46 WoS43 Europe PMC44 |
| 2021 | Sargeant, T. J., & Bensalem, J. (2021). Human autophagy measurement: an underappreciated barrier to translation. Trends in Molecular Medicine, 27(12), 1091-1094. Scopus10 WoS9 Europe PMC9 |
| 2021 | Carosi, J. M., Nguyen, T. N., Lazarou, M., Kumar, S., & Sargeant, T. J. (2021). ATG8ylation of proteins: a way to cope with cell stress?. The Journal of Cell Biology, 220(11), 1-4. Scopus18 WoS17 Europe PMC21 |
| 2020 | Xie, J., De Poi, S., Humphrey, S., Hein, L., Bruning, J., Pan, W., . . . Proud, C. (2020). TSC-Insensitive Rheb Mutations Induce Oncogenic Transformation Through a Combination of Hyperactive mTORC1 Signalling and Metabolic Reprogramming. |
| 2020 | Bensalem, J., Hattersley, K. J., Hein, L. K., Tong Teong, X., Carosi, J. M., Hassiotis, S., . . . Sargeant, T. J. (2020). Measurement of autophagic flux in humans: an optimized method for blood samples.. Autophagy, 17(10), 3238-3255. Scopus45 WoS45 Europe PMC48 |
| 2020 | Fourrier, C., Bryksin, V., Hattersley, K., Hein, L. K., Bensalem, J., & Sargeant, T. J. (2020). Comparison of chloroquine-like molecules for lysosomal inhibition and measurement of autophagic flux in the brain.. Biochem Biophys Res Commun, 534, 107-113. Scopus9 WoS8 Europe PMC10 |
| 2020 | Whyte, L. S., Hassiotis, S., Hattersley, K. J., Hemsley, K. M., Hopwood, J. J., Lau, A. A., & Sargeant, T. J. (2020). Lysosomal Dysregulation in the Murine AppNL-G-F/NL-G-F Model of Alzheimer's Disease. Neuroscience, 429, 143-155. Scopus16 WoS15 Europe PMC15 |
| 2019 | Mputhia, Z., Hone, E., Tripathi, T., Sargeant, T., Martins, R., & Bharadwaj, P. (2019). Autophagy modulation as a treatment of amyloid diseases. Molecules, 24(18), 3372-1-3372-20. Scopus52 WoS52 Europe PMC45 |
| 2019 | Cui, Y., Carosi, J. M., Yang, Z., Ariotti, N., Kerr, M. C., Parton, R. G., . . . Teasdale, R. D. (2019). Retromer has a selective function in cargo sorting via endosome transport carriers. Journal of Cell Biology, 218(2), 615-631. Scopus117 WoS111 Europe PMC121 |
| 2019 | Carosi, J. M., & Sargeant, T. J. (2019). Rapamycin and Alzheimer disease: a double-edged sword?. Autophagy, 15(8), 1460-1462. Scopus87 WoS83 Europe PMC74 |
| 2019 | Carosi, J. M., Hattersley, K. J., Cui, Y., Yang, Z., Teasdale, R. D., & Sargeant, T. J. (2019). Subcellular Fractionation of Hela Cells for Lysosome Enrichment Using a Continuous Percoll-Density Gradient. Bio Protocol, 9(18), 9 pages. Scopus10 WoS10 Europe PMC10 |
| 2018 | Hassiotis, S., Manavis, J., Blumbergs, P. C., Hattersley, K. J., Carosi, J. M., Kamei, M., & Sargeant, T. J. (2018). Lysosomal LAMP1 immunoreactivity exists in both diffuse and neuritic amyloid plaques in the human hippocampus. European Journal of Neuroscience, 47(9), 1043-1053. Scopus38 WoS36 Europe PMC31 |
| 2018 | Lloyd-Lewis, B., Krueger, C. C., Sargeant, T. J., D'Angelo, M. E., Deery, M. J., Feret, R., . . . Watson, C. J. (2018). Stat3-mediated alterations in lysosomal membrane protein composition. Journal of Biological Chemistry, 293(12), 4244-4261. Scopus27 WoS26 Europe PMC28 |
| 2018 | Gao, S., Casey, A. E., Sargeant, T. J., & Makinen, V. P. (2018). Genetic variation within endolysosomal system is associated with late-onset Alzheimer's disease. Brain, 141(9), 2711-2720. Scopus68 WoS67 Europe PMC68 |
| 2017 | Whyte, L. S., Lau, A. A., Hemsley, K. M., Hopwood, J. J., & Sargeant, T. J. (2017). Endo-lysosomal and autophagic dysfunction: a driving factor in Alzheimer's disease?. Journal of Neurochemistry, 140(5), 703-717. Scopus120 WoS121 Europe PMC113 |
| 2017 | Hein, L., Apaja, P., Hattersley, K., Grose, R., Xie, J., Proud, C., & Sargeant, T. (2017). A novel fluorescent probe reveals starvation controls the commitment of amyloid precursor protein to the lysosome. Biochimica et Biophysica Acta - Molecular Cell Research, 1864(10), 1554-1565. Scopus22 WoS20 Europe PMC21 |
| 2017 | Whyte, L. S., Hemsley, K. M., Lau, A. A., Hassiotis, S., Saito, T., Hopwood, J. J., & Sargeant, T. J. (2017). Reduction in open field activity in the absence of memory deficits in the AppNL-G-F knock-in mouse model of Alzheimer’s disease. Behavioural Brain Research, 336, 177-181. Scopus70 WoS68 Europe PMC67 |
| 2017 | Lloyd-Lewis, B., Sargeant, T. J., Kreuzaler, P. A., Resemann, H. K., Pensa, S., & Watson, C. J. (2017). Analysis of the involuting mouse mammary gland: An in vivo model for cell death. Methods in molecular biology (Clifton, N.J.), 1501, 165-186. Scopus6 Europe PMC5 |
| 2016 | Sargeant, T. J. (2016). Commentary: Possible involvement of lysosomal dysfunction in pathological changes of the brain in aged progranulin-deficient mice. Frontiers in Aging Neuroscience, 8(FEB), 3 pages. Scopus4 WoS3 Europe PMC1 |
| 2015 | Wooding, F. B. P., & Sargeant, T. J. (2015). Immunocytochemical Evidence for Golgi Vesicle Involvement in Milk Fat Globule Secretion. Journal of Histochemistry and Cytochemistry, 63(12), 943-951. Scopus11 WoS10 Europe PMC8 |
| 2014 | Sargeant, T. J., Lloyd-Lewis, B., Resemann, H. K., Ramos-Montoya, A., Skepper, J., & Watson, C. J. (2014). Stat3 controls cell death during mammary gland involution by regulating uptake of milk fat globules and lysosomal membrane permeabilization. Nature Cell Biology, 16(11), 1057-1068. Scopus139 WoS138 Europe PMC136 |
| 2014 | Pensa, S., Lloyd-Lewis, B., Sargeant, T. J., Resemann, H. K., Kahn, C. R., & Watson, C. J. (2014). Signal transducer and activator of transcription 3 and the phosphatidylinositol 3-kinase regulatory subunits p55α and p50α regulate autophagy in vivo. FEBS Journal, 281(20), 4557-4567. Scopus23 WoS20 Europe PMC21 |
| 2014 | Campbell, J. J., Botos, L. A., Sargeant, T. J., Davidenko, N., Cameron, R. E., & Watson, C. J. (2014). A 3-D in vitro co-culture model of mammary gland involution. Integrative Biology (United Kingdom), 6(6), 618-626. Scopus24 WoS24 Europe PMC20 |
| 2013 | Al-Lamki, R. S., Lu, W., Wang, J., Yang, J., Sargeant, T. J., Wells, R., . . . Bradley, J. R. (2013). TNF, acting through inducibly expressed TNFR2, drives activation and cell cycle entry of c-Kit+ cardiac stem cells in ischemic heart disease. Stem Cells, 31(9), 1881-1892. Scopus22 WoS21 Europe PMC20 |
| 2012 | Sargeant, T. J., Drage, D. J., Wang, S., Apostolakis, A. A., Cox, T. M., & Cachón-González, M. B. (2012). Characterization of Inducible Models of Tay-Sachs and Related Disease. Plos Genetics, 8(9), 15 pages. Scopus20 WoS16 Europe PMC14 |
| 2011 | Sargeant, T. J., Wang, S., Bradley, J., Smith, N. J. C., Raha, A. A., McNair, R., . . . Cachón-González, M. B. (2011). Adeno-associated virus-mediated expression of β-hexosaminidase prevents neuronal loss in the sandhoff mouse brain. Human Molecular Genetics, 20(22), 4371-4380. Scopus43 WoS40 Europe PMC38 |
| 2008 | Sargeant, T. J., Miller, J. H., & Day, D. J. (2008). Opioidergic regulation of astroglial/neuronal proliferation: Where are we now?. Journal of Neurochemistry, 107(4), 883-897. Scopus67 WoS57 Europe PMC52 |
| 2008 | Sargeant, T. J., Day, D. J., Miller, J. H., & Steel, R. W. J. (2008). Acute in utero morphine exposure slows G2/M phase transition in radial glial and basal progenitor cells in the dorsal telencephalon of the E15.5 embryonic mouse. European Journal of Neuroscience, 28(6), 1060-1067. Scopus27 WoS24 Europe PMC23 |
| 2007 | Sargeant, T. J., Day, D. J., Mrkusich, E. M., Foo, D. F., & Miller, J. H. (2007). Mu opioid receptors are expressed on radial glia but not migrating neuroblasts in the late embryonic mouse brain. Brain Research, 1175(1), 28-38. Scopus24 WoS24 Europe PMC18 |
| Year | Citation |
|---|---|
| 2025 | Tousian, H., Protzman, R. A., Sargeant, T. J., & Carosi, J. M. (2025). SenLect: a genetically encoded system to purify senescent cells. DOI |
| 2024 | Fourrier, C., Heilbronn, L., Teong, X. T., Gore, J., Sargeant, T., & Bensalem, J. (2024). Protocol for a randomized cross-over study measuring the effect of reduced protein intake on autophagic flux in healthy adults. DOI |
| 2024 | Carosi, J., Martin, A., Hein, L., Hassiotis, S., Hattersley, K., Turner, B., . . . Sargeant, T. (2024). Autophagy across tissues of aging mice. DOI |
| 2024 | Singh, S., Fourrier, C., Hattersley, K., Hein, L. K., Gore, J., Heilbronn, L. K., . . . Sargeant, T. J. (2024). A high protein meal does not change autophagy in human blood. DOI Europe PMC1 |
| 2024 | Dang, L. V. P., Martin, A., Carosi, J., Gore, J., Singh, S., & Sargeant, T. (2024). Cell-type specific autophagy in human leukocytes. DOI |
We have received funding from the following organisations:
NHMRC
BrightFocus
Diabetes Australia
The Rebecca L Cooper Medical Research Foundation
I teach in the following courses at the University of Adelaide:
Bachelor of Medical Studies (Medical Studies 3) - Ageing 1: Cellular Mechanisms and Ageing 9: Neurological Ageing (2023 - present)
PSYCHIAT 3200 (Fundamentals of Biological Psychiatry) - Biological mechanisms that underpin Alzheimer’s disease (2022 - present)
Biochemistry III (Cancer, Stem Cells and Development) - mTOR, autophagy and cancer (2018- present)
| Date | Role | Research Topic | Program | Degree Type | Student Load | Student Name |
|---|---|---|---|---|---|---|
| 2025 | Principal Supervisor | Creating a system to profile the molecular landscape of selective autophagy | Doctor of Philosophy | Doctorate | Full Time | Miss Sona Gopinathan |
| 2025 | Principal Supervisor | Creating a system to profile the molecular landscape of selective autophagy | Doctor of Philosophy | Doctorate | Full Time | Miss Sona Gopinathan |
| 2023 | Principal Supervisor | Lysosomal function in cellular senescence | Doctor of Philosophy | Doctorate | Full Time | Dr Hourieh Tousianshandiz |
| 2023 | Principal Supervisor | Lysosomal function in cellular senescence | Doctor of Philosophy | Doctorate | Full Time | Dr Hourieh Tousianshandiz |
| 2022 | Co-Supervisor | The Evaluation and Validation of Autophagy as a Novel Biomarker of Coronary Artery Disease | Doctor of Philosophy | Doctorate | Part Time | Dr Mau Tam Nguyen |
| 2022 | Co-Supervisor | The Evaluation and Validation of Autophagy as a Novel Biomarker of Coronary Artery Disease | Doctor of Philosophy | Doctorate | Part Time | Dr Mau Tam Nguyen |
| Date | Role | Research Topic | Program | Degree Type | Student Load | Student Name |
|---|---|---|---|---|---|---|
| 2019 - 2024 | Principal Supervisor | Beyond LC3-associated phagocytosis: cross-talk between autophagy and efferocytosis during microglial corpse clearance | Doctor of Philosophy | Doctorate | Full Time | Miss Sanjna Singh |
| 2018 - 2023 | Co-Supervisor | Investigation of the Endolysosomal Network in A Drosophila Model of Alzheimer’s Disease | Doctor of Philosophy | Doctorate | Full Time | Miss Sher Li Tan |
| 2015 - 2020 | Co-Supervisor | The Role of Heterozygous Lysosomal Storage Disorder Alleles as Risk Factors for Dementia | Doctor of Philosophy | Doctorate | Full Time | Ms Lauren Sue Whyte |
| Date | Role | Committee | Institution | Country |
|---|---|---|---|---|
| 2025 - ongoing | Chair | Institutional Biosafety Committee | SAHMRI | Australia |







