
APrf Michael Collins
School of Medicine
College of Health
Eligible to supervise Masters and PhD - email supervisor to discuss availability.
Dr Michael G. Collins MBChB FRACP PhD is a consultant nephrologist (kidney specialist) and clinical trialist working at the Royal Adelaide Hospital. He trained in Nephrology at Auckland City Hospital, New Zealand, and the Queen Elizabeth and Royal Adelaide Hospitals in Adelaide. From 2009-2013, he completed a PhD in transplant immunology with Professor Toby Coates at the University of Adelaide, while completing a number of clinical research projects including a multi-centre study of colorectal cancer screening in kidney transplant recipients. From this time onwards, he developed a strong interest in conducting high-quality clinical research with the goal of improving outcomes for kidney transplant recipients. From 2013-2021, Dr Collins worked as a consultant nephrologist at Auckland City Hospital, in New Zealand. From 2014 to 2018, he led a cross-disciplinary research group that completed a single-centre randomised controlled trial of nutrition interventions in kidney transplant recipients. In 2016, he proposed and ultimately became the co-lead investigator for the BEST-Fluids trial, a multi-centre, pragmatic, registry-based, investigator-initiated randomised controlled trial of IV fluid therapy in kidney transplant recipients. The trial was conducted as a collaboration between the Australasian Kidney Trials Network, the ANZDATA Registry and kidney transplant hospitals, clinicians and patients at 16 hospitals in Australia and New Zealand. The primary results of this study were published in the Lancet in 2023, and are expected to influence kidney transplant clinical practice worldwide, with further studies ongoing.Dr Collins relocated to work at the Royal Adelaide Hospital in 2022 where he is a senior nephrologist in the Central Northern Adelaide Renal and Transplantation Service (CNARTS), and a titleholder (clinical senior lecturer) at the University of Adelaide. He is the Deputy Chair of the Australasian Kidney Trials Network, and an active researcher in clinical trials. His ongoing research focuses on designing and conducting the future clinical trials of peri-operative interventions in kidney transplantation that will improve outcomes for patients.
Who am I and why do I work as a researcher in kidney disease and transplantation?
The BEST-Fluids trial
1. Australian Health Journal video article summarizing the BEST-Fluids trial
---
2. Listen to a podcast about the trial! The NephJC Freely Filtered Podcast discusses the impact of the trial and dissects the methods in detail.
https://www.nephjc.com/freelyfiltered/60/best-fluids
---
3. Lancet infographic of the key results
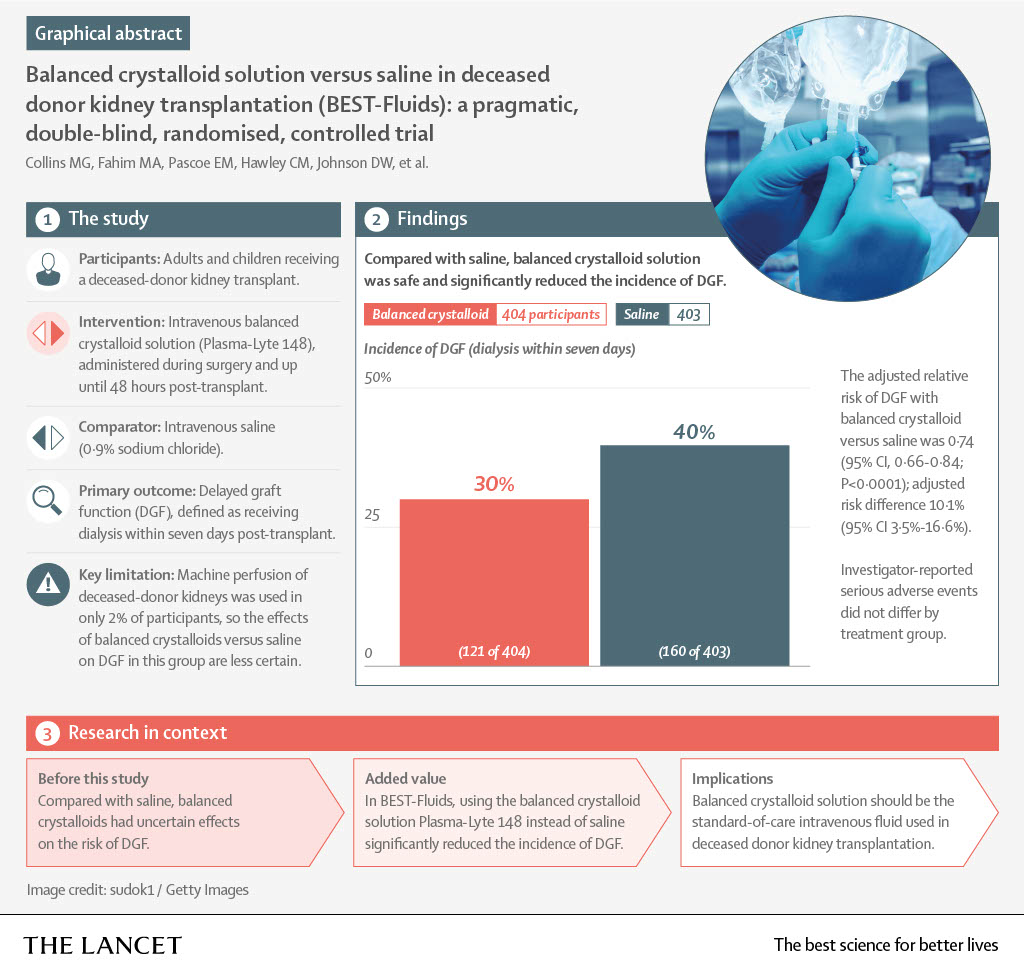
---
4. The key trial results summarised in a short video
---
5. Link to Australasian Kidney Trials Network website - https://aktn.org.au/best-fluids/
| Date | Position | Institution name |
|---|---|---|
| 2025 - ongoing | Clinical Associate Professor | University of Adelaide |
| 2022 - ongoing | Senior Consultant Nephrologist | Royal Adelaide Hospital |
| 2022 - 2025 | Clinical Senior Lecturer | University of Adelaide |
| 2017 - ongoing | Honorary Senior Fellow | University of Queensland |
| Date | Type | Title | Institution Name | Country | Amount |
|---|---|---|---|---|---|
| 2023 | Research Award | ANZSN Mid-Career Clinical Science Award | Australia and New Zealand Society of Nephrology | Australia | - |
| 2023 | Research Award | Kidney Health Australia Award for Best Clinical Research Presentation | Transplantation Society of Australia and New Zealand | Australia | - |
| 2021 | Distinction | Pass with Distinction | Harvard Medical School | United States | - |
| 2020 | Fellowship | Gavin and Ann Kellaway Medical Research Fellowship | Auckland Medical Research Foundation | New Zealand | NZD 37,000 |
| 2017 | Fellowship | Jacquot Research Establishment Fellowship | Royal Australasian College of Physicians | Australia | AUD 180,000 |
| 2011 | Research Award | Presidents Prize - Clinical | Transplantation Society of Australia and New Zealand | Australia | - |
| 2010 | Research Award | Young Investigator Award - Clinical Science | Australian and New Zealand Society of Nephrology | Australia | - |
| Date | Institution name | Country | Title |
|---|---|---|---|
| 2009 - 2013 | University of Adelaide | Australia | PhD |
| 1998 - 2000 | University of Auckland | New Zealand | MBChB |
| 1995 - 1997 | University of Auckland | New Zealand | Bachelor of Human Biology (BHB) |
| Date | Title | Institution | Country |
|---|---|---|---|
| 2021 - 2022 | Global Clinical Scholars Research Training Program 2021-2022 | Harvard Medical School | United States |
| 2001 - 2008 | FRACP | Royal Australasian College of Physicians | New Zealand |
| Year | Citation |
|---|---|
| 2026 | Barnett, D. R., & Collins, M. G. (2026). "See You in the Morning?" -Can Hypothermic Machine Perfusion Allow Longer Ischemic Times without Compromising Transplant Outcomes?. Clinical Journal of the American Society of Nephrology Cjasn, 21(1), 9-11. |
| 2026 | O'Reilly, C., Tunnicliffe, D., Blackley, A., Collins, M., O'Neill, E., Kim, S., . . . Francis, R. (2026). Corrigendum to “CARI Guideline: Evidence-Based Recommendations for Balanced Electrolyte Solutions to Improve Kidney Transplant Outcomes” (Kidney International Reports (2025) 10(8) (2566–2574), (S2468024925003559), (10.1016/j.ekir.2025.05.051)). Kidney International Reports, 11(3), 1 page. |
| 2025 | Logan, B., Pascoe, E. M., Viecelli, A. K., Johnson, D. W., Comans, T., Hawley, C. M., . . . Hubbard, R. E. (2025). Baseline Characteristics of Frailty and Disease Stage in Older People Living With CKD. Kidney International Reports, 10(1), 120-133. Scopus1 |
| 2025 | Chan, L. W., Irish, G. L., Goh, T. L., Alnasrallah, B., Davies, C. E., Sypek, M. P., . . . Collins, M. G. (2025). Outcomes of Living Kidney Donors Following Donor Nephrectomy in Aotearoa New Zealand. Kidney International Reports, 10(3), 762-771. |
| 2025 | Krishnasamy, R., Jardine, M. J., Sinha, S., Elder, G., Hawley, C., Toussaint, N., . . . Ruderman, I. (2025). Adaptive Designs for Clinical Trials in Nephrology. Journal of the American Society of Nephrology, 36(1), 147-149. Scopus5 Europe PMC6 |
| 2025 | Coghlan, D., Jaure, A., Hughes, A., Wu, R., Viecelli, A. K., Amir, N., . . . workshop investigators. (2025). Patient and caregiver involvement in implementing health research in chronic kidney disease: a workshop report. Clinical Kidney Journal, 18(3), sfaf021-1-sfaf021-5. |
| 2025 | Chan, L. W., Irish, G. L., Goh, T. L., Alnasrallah, B., Davies, C. E., Sypek, M. P., . . . Collins, M. G. (2025). Corrigendum to “Outcomes of Living Kidney Donors Following Donor Nephrectomy in Aotearoa New Zealand” [Volume 10, Issue 3, March 2025, Pages 762-771] (Kidney International Reports (2025) 10(3) (762–771), (S2468024924033849), (10.1016/j.ekir.2024.11.1362)). Kidney International Reports, 10(5), 1605-1606. |
| 2025 | Venkataraman, K., Irish, G. L., Collins, M. G., & Clayton, P. A. (2025). The Association Between Early Graft Function, Donor Type and Long-Term Kidney Transplant Outcomes. Transplant International, 38, 14197-1-14197-11. Scopus2 WoS2 Europe PMC2 |
| 2025 | Stretton, B., Venkataraman, K., Kovoor, J., Gupta, A., Bacchi, S., Liew, D., . . . Boyd, M. (2025). Characterizing Perioperative Sleep in Acute Renal Transplants.. Prog Transplant, 35(3), 15269248251349760. |
| 2024 | Wan, S. S., Wyburn, K., Chadban, S. J., & Collins, M. G. (2024). Balanced Electrolyte Solutions Versus 0.9% Saline for Kidney Transplantation: An Updated Systematic Review and Meta-analysis. Transplantation Direct, 11(1), e1687. Scopus2 WoS2 Europe PMC2 |
| 2024 | Mulley, W. R., Hughes, P. D., Collins, M. G., Pilmore, H. L., Clayton, P. A., Wyld, M. L., . . . Lim, W. H. (2024). Defining causes of death-censored kidney allograft failure: A 5-year multicentre ANZDATA and clinical cross-sectional study. Nephrology, 29(12), 930-940. Scopus1 WoS1 Europe PMC2 |
| 2024 | Collins, M. G., & Chadban, S. J. (2024). Dealing with Delayed Graft Function. Transplantation, 108(6), 1273-1274. Scopus2 WoS2 Europe PMC2 |
| 2024 | Venkataraman, K., McTaggart, S. J., & Collins, M. G. (2024). Choosing fluids to reduce the risks of acute electrolyte disturbances in children after a kidney transplant. Kidney International, 105(2), 247-250. Scopus1 WoS1 |
| 2024 | Collins, M. G., Fahim, M. A., Hawley, C. M., Johnson, D. W., & Chadban, S. J. (2024). Questions about the BEST-Fluids trial - Authors' reply. The Lancet, 403(10430), 911-912. Scopus1 Europe PMC1 |
| 2024 | Collins, M. G., Fahim, M. A., Hawley, C. M., Johnson, D. W., & Chadban, S. (2024). Authors' reply. LANCET, 403(10430), 911-912. WoS1 |
| 2024 | Collins, M. G., Hawley, C. M., & McDonald, S. P. (2024). Nephrology Clinical Trials in Learning Health Systems. Journal of the American Society of Nephrology, 35(9), 1274-1277. |
| 2023 | Logan, B., Viecelli, A., Johnson, D., Aquino, E., Bailey, J., Comans, T., . . . Yip, B. (2023). Study protocol for The GOAL Trial: comprehensive geriatric assessment for frail older people with chronic kidney disease to increase attainment of patient-identified goals—a cluster randomised controlled trial. Trials, 24(1), 1-15. Scopus18 WoS18 Europe PMC18 |
| 2023 | Collins, M. G., Fahim, M. A., Pascoe, E. M., Hawley, C. M., Johnson, D. W., Varghese, J., . . . Australasian Kidney Trials Network. (2023). Balanced crystalloid solution versus saline in deceased donor kidney transplantation (BEST-Fluids): a pragmatic, double-blind, randomised, controlled trial. The Lancet, 402(10396), 105-117. Scopus67 WoS60 Europe PMC52 |
| 2022 | Pascoe, E. M., Chadban, S. J., Fahim, M. A., Hawley, C. M., Johnson, D. W., & Collins, M. G. (2022). Statistical analysis plan for Better Evidence for Selecting Transplant Fluids (BEST-Fluids): a randomised controlled trial of the effect of intravenous fluid therapy with balanced crystalloid versus saline on the incidence of delayed graft function in deceased donor kidney transplantation. Trials, 23(1), 52-1-52-4. Scopus3 WoS2 Europe PMC3 |
| 2022 | Lim, W. H., Ooi, E., Pilmore, H. L., Johnson, D. W., McDonald, S. P., Clayton, P., . . . Wong, G. (2022). Interactions between donor age and 12-month estimated glomerular filtration rate on allograft and patient outcomes after kidney transplantation. Transplant International, 36, 10199-1-10199-10. Scopus8 WoS7 Europe PMC5 |
| 2022 | Pascoe, E. M., Chadban, S. J., Fahim, M. A., Hawley, C. M., Johnson, D. W., & Collins, M. G. (2022). Correction to: Statistical analysis plan for better evidence for selecting transplant fluids (BEST-fluids): a randomised controlled trial of the effect of intravenous fluid therapy with balanced crystalloid versus saline on the incidence of delayed graft function in deceased donor kidney transplantation (Trials, (2022), 23, 1, (52), 10.1186/s13063-021-05989-w). Trials, 23(1), 1 page. |
| 2022 | Collins, M. G., Fahim, M. A., Pascoe, E. M., Hawley, C. M., Johnson, D. W., Varghese, J., . . . Chadban, S. J. (2022). Baseline Characteristics and Representativeness of Participants in the BEST-Fluids Trial: A Randomized Trial of Balanced Crystalloid Solution Versus Saline in Deceased Donor Kidney Transplantation. Transplantation Direct, 8(12), e1399-1-e1399-13. Scopus8 WoS8 Europe PMC7 |
| 2022 | Viecelli, A. K., Teixeira-Pinto, A., Valks, A., Baer, R., Cherian, R., Cippà, P. E., . . . Jenkins, S. (2022). Study protocol for Vascular Access outcome measure for function: a vaLidation study In hemoDialysis (VALID): A multi-center, multinational validation study to assess the accuracy and feasibility of measuring vascular access function in clinical practice. BMC Nephrology, 23(1), 372-1-372-12. Scopus3 WoS3 Europe PMC3 |
| 2021 | Pei, J., Cho, Y., See, Y. P., Pascoe, E. M., Viecelli, A. K., Francis, R. S., . . . Johnson, D. W. (2021). Impact of deceased donor with acute kidney injury on subsequent kidney transplant outcomes-an ANZDATA registry analysis. Plos One, 16(3 March), 19 pages. Scopus16 WoS15 Europe PMC12 |
| 2021 | Weinberg, L., Collins, M. G., & Peyton, P. (2021). Urine the Right Direction: The Consensus Statement from the Committee on Transplant Anesthesia of the American Society of Anesthesiologists on Fluid Management during Kidney Transplantation. Transplantation, 105(8), 1655-1657. Scopus3 WoS1 |
| 2021 | Lim, W. H., Adams, B., Alexander, S., Bouts, A. H. M., Claas, F., Collins, M., . . . Wong, G. (2021). Improve in-depth immunological risk assessment to optimize genetic-compatibility and clinical outcomes in child and adolescent recipients of parental donor kidney transplants: protocol for the INCEPTION study. BMC Nephrology, 22(1), 1-11. Scopus3 WoS3 Europe PMC1 |
| 2020 | Wright, R. S., Collins, M. G., Stoekenbroek, R. M., Robson, R., Wijngaard, P. L. J., Landmesser, U., . . . Kallend, D. (2020). Effects of Renal Impairment on the Pharmacokinetics, Efficacy, and Safety of Inclisiran: An Analysis of the ORION-7 and ORION-1 Studies. Mayo Clinic Proceedings, 95(1), 77-89. Scopus134 WoS113 Europe PMC99 |
| 2020 | Collins, M. G., Fahim, M. A., Pascoe, E. M., Dansie, K. B., Hawley, C. M., Clayton, P. A., . . . BEST-Fluids Investigators and the Australasian Kidney Trials Network. (2020). Study Protocol for Better Evidence for Selecting Transplant Fluids (BEST-Fluids): a pragmatic, registry-based, multi-center, double-blind, randomized controlled trial evaluating the effect of intravenous fluid therapy with Plasma-Lyte 148 versus 0.9% saline on delayed graft function in deceased donor kidney transplantation. Trials, 21(1), 428-1-428-19. Scopus19 WoS15 Europe PMC13 |
| 2020 | Htay, H., Pascoe, E. M., Hawley, C. M., Campbell, S. B., Chapman, J., Cho, Y., . . . Johnson, D. W. (2020). Patient and center characteristics associated with kidney transplant outcomes: a binational registry analysis. Transplant International, 33(12), 1667-1680. Scopus8 WoS7 Europe PMC5 |
| 2019 | Collins, M. G., Symonds, E. L., Bampton, P. A., & Coates, P. T. (2019). Fecal immunochemical screening for advanced colorectal neoplasia in patients with CKD: Accurate or not?. Journal of the American Society of Nephrology, 30(11), 2275. Scopus1 Europe PMC1 |
| 2019 | See, E. J., Hawley, C. M., Cho, Y., Toussaint, N. D., Agar, J. W. M., Pascoe, E. M., . . . Johnson, D. W. (2019). Comparison of graft and patient outcomes following kidney transplantation in extended hour and conventional haemodialysis patients. Nephrology, 24(1), 111-120. Scopus1 WoS1 Europe PMC1 |
| 2018 | Henggeler, C. K., Plank, L. D., Ryan, K. J., Gilchrist, E. L., Casas, J. M., Lloyd, L. E., . . . Collins, M. G. (2018). A Randomized Controlled Trial of an Intensive Nutrition Intervention Versus Standard Nutrition Care to Avoid Excess Weight Gain After Kidney Transplantation: The INTENT Trial. Journal of Renal Nutrition, 28(5), 340-351. Scopus51 WoS44 Europe PMC37 |
| 2018 | Kosloski, M. P., Zhao, W., Marbury, T. C., Preston, R. A., Collins, M. G., Pugatch, D., . . . Liu, W. (2018). Effects of Renal Impairment and Hemodialysis on the Pharmacokinetics and Safety of the Glecaprevir and Pibrentasvir Combination in Hepatitis C Virus-Negative Subjects. Antimicrobial Agents and Chemotherapy, 62(3), 10 pages. Scopus20 WoS18 Europe PMC15 |
| 2017 | Weinberg, L., Harris, L., Bellomo, R., Ierino, F. L., Story, D., Eastwood, G., . . . Mount, P. F. (2017). Effects of intraoperative and early postoperative normal saline or Plasma-Lyte 148 ® on hyperkalaemia in deceased donor renal transplantation: A double-blind randomized trial. British Journal of Anaesthesia, 119(4), 606-615. Scopus71 WoS58 Europe PMC54 |
| 2015 | Collins, M. G., & Clayton, P. A. (2015). Thai transplant registry: An important resource for the Asia Pacific region. Nephrology, 20(4), 227-228. |
| 2014 | Collins, M. G., Rogers, N. M., Jesudason, S., Kireta, S., Brealey, J., & Coates, P. T. (2014). Spontaneous glomerular mesangial lesions in common marmoset monkeys (Callithrix jacchus): a benign non-progressive glomerulopathy. Journal of Medical Primatology, 43(6), 477-487. Scopus16 WoS15 Europe PMC12 |
| 2014 | Ryan, K. J., Segedin Casas, J. M., Mash, L. E., McLellan, S. L., Lloyd, L. E., Stinear, J. W., . . . Collins, M. G. (2014). The effect of intensive nutrition interventions on weight gain after kidney transplantation: Protocol of a randomised controlled trial. BMC Nephrology, 15(1), 9 pages. Scopus23 WoS24 Europe PMC20 |
| 2012 | Collins, M., Teo, E., Cole, S., Chan, C., McDonald, S., Russ, G., . . . Coates, P. (2012). Screening for colorectal cancer and advanced colorectal neoplasia in kidney transplant recipients: cross sectional prevalence and diagnostic accuracy study of faecal immunochemical testing for haemoglobin and colonoscopy. BMJ (Online), 345(7871), 1-14. Scopus46 WoS39 Europe PMC34 |
| 2012 | Jesudason, S., Collins, M., Rogers, N., Kireta, S., & Coates, P. (2012). Non-human primate dendritic cells. Journal of Leukocyte Biology, 91(2), 217-228. Scopus13 WoS11 Europe PMC12 |
| 2009 | Collins, M., Chang, S., Russ, G., & McDonald, S. (2009). Outcomes of Transplantation Using Kidneys From Donors Meeting Expanded Criteria in Australia and New Zealand, 1991 to 2005. Transplantation, 87(8), 1201-1209. Scopus32 WoS30 Europe PMC27 |
| 2009 | Collins, M. G., Brunskill, A. J., McDonald, S. P., Collins, J. F., & Dittmer, I. D. (2009). KIDNEY TRANSPLANT OUTCOMES AMONG THE MAORI AND PACIFIC PEOPLES OF NEW ZEALAND. NEPHROLOGY, 14, A56. |
| 2007 | Collins, M. G., Chang, S. H., Mcdonald, S. P., & Russ, G. R. (2007). EXPANDED CRITERIA DONORS FOR KIDNEY TRANSPLANTATION: IS THE MODEL FOR ASSESSMENT OF INCREASED RISK OF POOR OUTCOME VALID FOR AUSTRALIA AND NEW ZEALAND?. NEPHROLOGY, 12, A18. |
| 2007 | Collins, M. G., Chang, S. H., Mcdonald, S. P., & Russ, G. R. (2007). DECEASED DONOR KIDNEY TRANSPLANTATION: WHAT ARE THE EFFECTS OF DONOR FACTORS ON GRAFT OUTCOME IN AUSTRALIA AND NEW ZEALAND?. NEPHROLOGY, 12, A19. WoS1 |
| 1999 | Collins, M. G. (1999). Medical students and debt: A survey of students at the School of Medicine, University of Auckland. New Zealand Medical Journal, 112(1085), 123-126. Scopus5 WoS5 |
| Year | Citation |
|---|---|
| 2022 | Collins, M. G., Fahim, M., Pascoe, E., Clayton, P. A., Hawley, C., Johnson, D. W., . . . Chadban, S. J. (2022). Baseline Characteristics and Representativeness of the BEST-Fluids Trial Participants: A Randomized Trial of Balanced Crystalloid Solution vs. Saline in Deceased Donor Kidney Transplantation. In JOURNAL OF THE AMERICAN SOCIETY OF NEPHROLOGY Vol. 33 (pp. 971). AMER SOC NEPHROLOGY. |
| 2017 | See, E., Hawley, C., Cho, Y., Toussaint, N., Agar, J., Pascoe, E., . . . Johnson, D. (2017). A COMPARISON OF GRAFT AND PATIENT OUTCOMES FOLLOWING KIDNEY TRANSPLANTATION IN EXTENDED HOUR AND CONVENTIONAL HAEMODIALYSIS PATIENTS. In NEPHROLOGY DIALYSIS TRANSPLANTATION Vol. 32 (pp. 2 pages). OXFORD UNIV PRESS. |
| 2011 | Collins, M. G., Bampton, P. A., Young, G. P., Cole, S. R., McDonald, S. P., Russ, G. R., & Coates, P. T. (2011). Colorectal Cancer and Pre-Malignant Colorectal Neoplasia Are Detected with Increased Prevalence in Prospectively Screened Asymptomatic Kidney Transplant Recipients and May Be Linked to Viral Infection. In AMERICAN JOURNAL OF TRANSPLANTATION Vol. 11 (pp. 510). Philadelphia, PA: WILEY-BLACKWELL. |
| 2010 | Collins, M. G., Teo, E., Chan, C., Young, G. P., Cole, S. R., McDonald, S. P., . . . Coates, P. T. (2010). Screening for Colorectal Cancer in Kidney Transplant Recipients: A Prospective Cross-Sectional Study of Faecal Immunochemical Testing Versus Colonoscopy. In AMERICAN JOURNAL OF TRANSPLANTATION Vol. 10 (pp. 472). San Diego, CA: WILEY-BLACKWELL PUBLISHING, INC. |
| Year | Citation |
|---|---|
| 2013 | Collins, M. (2013). Developing a non-human primate model of dendritic cell based immunotherapy in transplantation: Studies in the common marmoset monkey (Callithrix jacchus). (PhD Thesis, University of Adelaide). |
- New Zealand Health Research Council (HRC) Project Grant 2019: 19/268. Serum phosphate to improve outcomes for dialysis patients: The PHOSPHATE trial. NZD$1,266,603 [Named Investigator]
- Australian Government Medical Research Future Fund 2018: Rare Cancers, Rare Diseases and Unmet Needs Clinical Trials Program Grant: APP1152390. The BEST Fluids study. AUD$1,117,150 [Chief Investigator B]
- HRC Project Grant 2017: 17/414. The BEST Fluids study. NZD$549,035 [First Named Investigator/CIA]
- Royal Australasian College of Physicians. Jacquot Research Establishment Fellowship Grant 2017 and 2018. AUD $180,000.
- Better Evidence and Translation Chronic Kidney Disease (BEAT-CKD) grant 2016-7. Seed funding for The BEST Fluids study. AUD$200,000
- Auckland District Health Board Charitable Trust – Project grant 2015. Outcomes for Live Kidney Donors following Nephrectomy in Aotearoa New Zealand: the LIVE DONATE NZ study. NZD $30,000.00
- Auckland District Health Board Charitable Trust – Project grant 2013. Optimal nutrition intervention to prevent excessive weight gain post kidney transplantation: pilot randomised controlled trial. NZD $30,000.00
- ANZSN-Amgen Quality Assurance Grants 2014. Optimal nutrition intervention to prevent excessive weight gain post kidney transplantation: pilot randomised controlled trial. AUD $10,000.00
- CellCept Australia Research Grants, 2008. Screening for colorectal cancer in recipients of kidney transplants. AUD $20,000.00
- The Queen Elizabeth Hospital Research Foundation Research Grants, 2010. Screening for colorectal cancer in recipients of kidney transplants. AUD $10,000.00
- Accredited supervisor of Advanced trainees in Nephrology (Royal Australasian College of Physicians)
- Lecturer for the University of Adelaide Bachelor of Medical Studies and Doctor of Medicine (BMD) program
| Date | Role | Research Topic | Program | Degree Type | Student Load | Student Name |
|---|---|---|---|---|---|---|
| 2023 | Principal Supervisor | Peri-operative Management of Kidney Transplantation | Doctor of Philosophy | Doctorate | Full Time | Mr Karthik Venkataraman |
| 2023 | Principal Supervisor | Peri-operative Management of Kidney Transplantation | Doctor of Philosophy | Doctorate | Full Time | Mr Karthik Venkataraman |
| Date | Role | Research Topic | Location | Program | Supervision Type | Student Load | Student Name |
|---|---|---|---|---|---|---|---|
| 2021 - 2022 | Co-Supervisor | Obesity and Access to Kidney Transplantation | University of Auckland | Master of Nursing | Master | Part Time | Peta Kelly |
| 2020 - 2021 | External Supervisor | Thymoglobuline and the risk of delayed graft function after kidney transplantation | University of Queensland | Master of Pharmacy | Master | Part Time | Emily McCulloch |
| 2016 - 2017 | Co-Supervisor | Intensive Nutrition interventions to prevent weight after kidney transplantation | University of Auckland | Master of Health Science | Master | Full Time | Cordula Henggeler |
| 2015 - 2015 | Co-Supervisor | Intensive Nutrition interventions to prevent weight after kidney transplantation | University of Auckland | Master of Health Science | Master | Full Time | Emily Gilchrist |
| 2014 - 2014 | Co-Supervisor | Intensive Nutrition interventions to prevent weight after kidney transplantation | University of Auckland | Master of Health Science | Master | Full Time | Kristin Ryan |
| Date | Role | Committee | Institution | Country |
|---|---|---|---|---|
| 2020 - ongoing | Vice-Chair | Australasian Kidney Trials Network (AKTN) Scientific Committee | University of Queensland | Australia |
| 2020 - 2021 | Advisory Board Member | National Renal Advisory Board | Ministry of Health, New Zealand Government | New Zealand |
| 2017 - 2021 | Member | Australia and New Zealand Dialysis and Transplant Registry - Advisory Committee | South Australian Health and Medical Research Institute | Australia |
| 2015 - 2021 | Member | Research Review Committee | Auckland District Health Board | New Zealand |
| Date | Role | Membership | Country |
|---|---|---|---|
| 2022 - ongoing | Member | Australian Clinical Trials Alliance | Australia |
| 2008 - ongoing | Member | The Transplantation Society | Canada |
| 2008 - ongoing | Member | American Society of Transplantation | United States |
| 2007 - ongoing | Member | International Society of Nephrology | Belgium |
| 2006 - ongoing | Member | American Society of Nephrology | United States |
| 2005 - ongoing | Member | Transplantation Society of Australia and New Zealand | Australia |
| 2005 - ongoing | Member | Australia and New Zealand Society of Nephrology | Australia |
| Date | Role | Editorial Board Name | Institution | Country |
|---|---|---|---|---|
| 2017 - 2021 | Board Member | Nephrology | Asia Pacific Society of Nephrology | Australia |






