Dr Jenna Crowe-Riddell
ARC Grant-Funded Research Fellow
School of Biological Sciences
College of Sciences
Eligible to supervise Masters and PhD - email supervisor to discuss availability.
My research focuses on the evolution senses and brains in reptiles. I like to take an integrative approach by using morpological, molecular and behavioural data to understand the evolution of complex sensory traits.My current projects include an ARC Discovery Project investigating brain evolution in Australian squamates (snakes and lizards). This project aims uses diffusible iodine contrast-enhanced CT scanning to capture changes in brain shape and size during transitions to new ecologies/biomes. This research dovetails with development projects on how incubation temperature is impacting brain development in thermo-sensitive species like bearded dragons. My current ARC DECRA project investigates the mechanisms underlying sensory adaptations in snakes. This research will harness innovative phenotypic imaging and genomic sequencing, to study the coordinated changes among sensory systems in a range of ecologically diverse snakes. Expected outcomes include a large database of 3D digital anatomical models from Australian and international museum collections, and new knowledge on the genetic processes influencing sensory receptor evolution in vertebrates.These projects have benefits for conservation by using neural adaptability as a framework for estimating potential extinction risk for vulnerable species. I am also passionate about conservation in sea snakes, helping to create an environmental DNA (eDNA) assay for detecting endangered sea snake species in Western Australian Marine Parks.

About me
I am an ARC DECRA fellow at the University of Adelaide in the School of Biological Sciences. My research focuses on the evolution senses and brains in reptiles. I like to take an integrative approach by using morphological, molecular and behavioural data to understand the evolution of complex sensory traits.
I completed my PhD in Ecology and Evolution at the University of Adelaide in 2019 focusing on "Cutaneous senses in marine snakes". After PhD, I embarked on my first postdoc position in Ann Arbor at the University of Michigan in the Davis Rabosky Lab working on evolution, morphology and transcriptomics in neotropical snakes, and had the privilege of working in the incredible collections at the University of Michigan Museum of Zoology. During the pandemic, I got "stuck" back in Australia and worked remotely until my next postdoc position in the Neuroecology Lab led by Shaun Collin at La Trobe University in Melbourne. Here, I expanded my skills in neuroanatomy and behaviour working on a range of marine organisms including fishes and sharks. In 2024, I moved back to Adelaide to start my ARC DECRA fellowship where I am building my lab, focusing on brain evolution and sensory ecology in reptiles.
Research in the lab
Current projects include an ARC Discovery Project investigating brain evolution in Australian squamates (snakes and lizards). This project aims uses diffusible iodine contrast-enhanced CT scanning to capture changes in brain shape and size during transitions to new ecologies/biomes. This research dovetails with development projects on how incubation temperature is impacting brain development in thermo-sensitive species like bearded dragons.
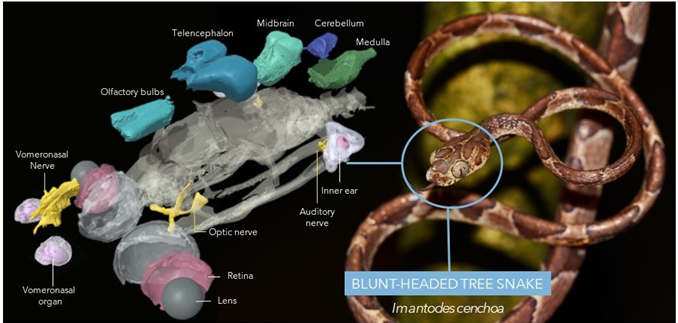
My current ARC DECRA project investigates the mechanisms underlying sensory adaptations in snakes. This research will harness innovative phenotypic imaging and genomic sequencing, to study the coordinated changes among sensory systems in a range of ecologically diverse snakes. Check out the interactive "exploding snake brain" below (and more interactive models here). Expected outcomes include a large database of 3D digital anatomical models from Australian and international museum collections, and new knowledge on the genetic processes influencing sensory receptor evolution in vertebrates.
Exploding snake brain 🐍🧠💥 by Michigan Herpetology (UMMZ) on Sketchfab
Understanding sensory morphology can tell us about how animals are interacting with their environments, but a key missing part is behaviour. Recent projects are delving into snake (and crocodile!) behaviour. We are conducting behavioural experiments on sea snakes to better understand their sensory abilities, and link this with gene expression and neuroanatomical approaches to build a complete picture of a sea snake's sensory world. I am currently collaborating with the Minderoo Foundation Exmouth Research Laboratory where we have the opportunity to collect and test wild snakes in captivity, an incredible opportunity to observe these enigmatic animals.

These projects have benefits for conservation by using neural adaptability as a framework for estimating potential extinction risk for vulnerable species. They also have great potential for fostering our curiosity about the living world. Something that I am very passionate about.
I also co-supervise projects conservation in sea snakes, where we are currently creating an environmental DNA (eDNA) assay for detecting endangered sea snake species in Western Australian as part of ongoing projects with Australian Marine Parks.
Student Projects
Project 1 (Honours): Serpent sensory innovation in the evolutionary transition from land to sea Snakes are incredibly successful at living in aquatic habitats, which imposes major challenges on sensory systems that must attune to a range of new/altered stimuli underwater. This project aims to understand how senses such as mechanoreception and chemoreception has evolved in snakes as they evolved from an air to water-based medium. This project will use a genome to phenome approach to understand how senses have elaborated/degenerated as snakes transitioned from land to sea. This project will provide new insights into the sensory ecology of sea snakes, as well as how genotypes can promote/constrain sensory phenotypic change across vertebrates. Although sea snakes are one of the main groups I study, this project is flexible could instead/also focus on other evolutionary transitions in snakes (arboreal, burrowing) or other reptiles.
Snakes are incredibly successful at living in aquatic habitats, which imposes major challenges on sensory systems that must attune to a range of new/altered stimuli underwater. This project aims to understand how senses such as mechanoreception and chemoreception has evolved in snakes as they evolved from an air to water-based medium. This project will use a genome to phenome approach to understand how senses have elaborated/degenerated as snakes transitioned from land to sea. This project will provide new insights into the sensory ecology of sea snakes, as well as how genotypes can promote/constrain sensory phenotypic change across vertebrates. Although sea snakes are one of the main groups I study, this project is flexible could instead/also focus on other evolutionary transitions in snakes (arboreal, burrowing) or other reptiles.
Project 2 (Honours, Masters or PhD): Brain adaptations to changing environments in snakes and lizards
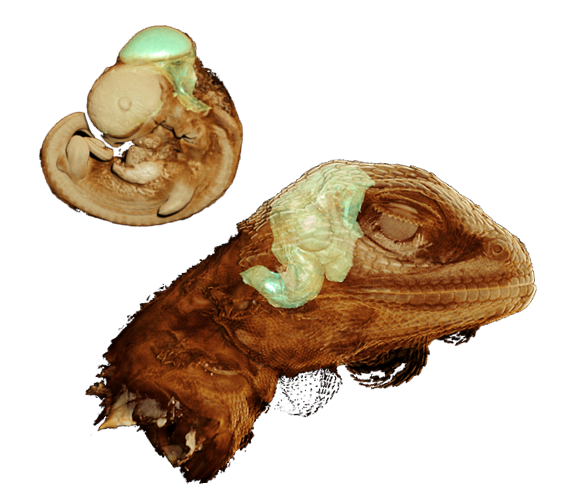
This project aims to understand how the brains of Australia’s snakes and lizards have changed in response to past and ongoing environmental change. Brains crucially underpin the behavioural adaptations needed to cope with environmental change. Yet, studies of evolutionary adaptation have rarely assessed brains, especially in reptiles. Furthermore, climate change is predicted to impact developing brains especially in thermosensitive species like reptiles. The student will develop skills in microCT scanning and morphometrics to quantify neuroanatomy in reptiles (e.g., elapids, dragons, goannas) located in Australian museum collections and/or immunohistochemistry and neuronal staining methods to look at embryonic brain change in response to hot-incubation temperatures in bearded dragons (Pogona vitticeps).
If you'd like to find out more about the lab - please reach out.
Select publications
- Wagner, A, Johnson, C, Ha, MH, Sanders, KL, Collin, SP, Crowe-Riddell, JM (2025) Morphological evidence for a directional flow mechanoreceptor in olive-headed sea snakes (Hydrophis major). Journal of Morphology 286:e70093. http://dx.doi.org/10.1002/jmor.70093
- Folwell, M, Sanders, KL, Brennan, PLR, Crowe-Riddell, JM (2022) First evidence of hemiclitores in snakes. Proceedings of the Royal Society B 289: 20221702. DOI: https://doi.org/10.1098/rspb.2022.1702
- Crowe-Riddell, JM, Callahan, S, Nagesan, RS, Gray, JA, Davis Rabosky, AR (2021) A guide for optimal iodine staining and high-throughput diceCT scanning in snakes. Ecology and Evolution 11: 11587–11603.3 DOI: https://doi/org/10.1002/ece3.7467
- Crowe-Riddell, JM, Simões, BR, Partridge, JC, Schwerdt, JG, Breen, J, Ludington, A, Gower, DG, Sanders, KL (2019) Phototactic tails: Evolution and molecular basis of a novel sensory trait in sea snakes. Molecular Ecology 28, 2013–2028. DOI: https://doi.org/10.1111/mec.15022
- Crowe-Riddell, JM, Snelling, EP, Watson, A, Kyuseop Suh, A, Partridge, JC, Sanders, KL (2016) The evolution of scale sensilla in the transition from land to sea in elapid snakes. Royal Society Open Biology 2016, 160054. DOI: https://doi.org/10.1098/rsob.160054
Select media
- Watch our episode of "Dr Ann's secret lives of sea snakes" with the Sanders Lab!
- The Atlantic, “Surprise! Snakes have clitorises”, Katherine J. Wu, 14 Dec 2022
- New York Times, “Scientists overlooked the snake clitoris, until now”, Alex Fox, 14 Dec 2022
- Australian Museum Blog, “The sex life aquatic: How sea snakes have overcome the tricks of sex at sea” June 29 2021
- Environment Institute Blog, “Beyond the elevator pitch: How do we engage people in science?” Dec 27 2018
- Australian Geographic, “Sea snakes’ sixth sense”, Georgie Meredith, 14 June 2016
My profile by the Department for Innovation and Skills when I was a finalised for the 2019 Science Awards.
| Date | Position | Institution name |
|---|---|---|
| 2024 - ongoing | ARC DECRA Fellow | University of Adelaide |
| 2021 - 2024 | Postdoctoral Researcher | La Trobe University |
| 2019 - 2021 | Postdoctoral Researcher | University of Michigan |
| 2016 - ongoing | Adminstrative assistant | University of Adelaide |
| 2015 - ongoing | PhD | University of Adelaide |
| Date | Type | Title | Institution Name | Country | Amount |
|---|---|---|---|---|---|
| 2016 | Scholarship | Fulbright Postgraduate Scholarship | - | - | - |
| Date | Institution name | Country | Title |
|---|---|---|---|
| 2015 - 2019 | University of Adelaide | Australia | PhD |
| 2014 - 2014 | University of Adelaide | Australia | Ba of Science (1st Class Honours) |
| 2008 - 2011 | Australian National University | Australia | Ba of Science |
| Year | Citation |
|---|---|
| 2025 | Sherratt, E., Crowe-Riddell, J., Palci, A., Ammresh., Hutchinson, M. N., Lee, M. S. Y., & Sanders, K. L. (2025). Rapid evolution and cranial morphospace expansion during the terrestrial to marine transition in elapid snakes. Evolution, 79(12), 1-14. |
| 2025 | Wagner, A., Johnson, C., Ha, M. H., Sanders, K. L., Collin, S. P., & Crowe-Riddell, J. M. (2025). Morphological Evidence for a Directional Flow Mechanoreceptor in Olive-Headed Sea Snakes (Hydrophis major). Journal of Morphology, 286(10), e70093-1-e70093-22. |
| 2025 | Coppersmith, S., Goiran, C., Sanders, K. L., Crowe-Riddell, J. M., Chateau, O., Shine, R., & Udyawer, V. (2025). Wiggle and glide: fine-scale telemetry reveals unique diving strategies in benthic-foraging sea snakes. Movement Ecology, 13(1), 62-1-62-17. Scopus1 WoS1 Europe PMC1 |
| 2025 | Collin, H. B., Ha, M. H., Wagner, A., Folwell, M., Dunstan, N., Crowe-Riddell, J., & Collin, S. P. (2025). Surface Topography and Ultrastructure of the Spectacular Cells in the Eyes of Land and Sea Snakes (Squamata, Reptilia): Functional Adaptations of Micro-Ornamentation. Journal of Morphology, 286(9), e70084-1-e70084-27. |
| 2024 | Collin, S. P., Yopak, K. E., Crowe-Riddell, J. M., Camilieri-Asch, V., Kerr, C. C., Robins, H., . . . Chapuis, L. (2024). Bioimaging of sense organs and the central nervous system in extant fishes and reptiles in situ: A review. Anatomical Record, 1-27. Scopus6 WoS6 Europe PMC4 |
| 2024 | Crowe-Riddell, J. M., Zdenek, C. N., Sanders, K. L., & Rasmussen, A. R. (2024). Sea snakes. Current biology : CB, 34(17), R806-R807. Scopus2 WoS2 Europe PMC3 |
| 2023 | Srodawa, K., Cerda, P. A., Davis Rabosky, A. R., & Crowe-Riddell, J. M. (2023). Evolution of Three-Finger Toxin Genes in Neotropical Colubrine Snakes (Colubridae). Toxins, 15(9), 16 pages. Scopus5 WoS4 Europe PMC3 |
| 2023 | Palci, A., Lee, M. S. Y., Crowe-Riddell, J., & Sherratt, E. (2023). Shape and size variation in elapid snake fangs, and the effects of phylogeny and diet. Evolutionary Biology, 50(4), 476-487. Scopus5 WoS5 |
| 2022 | Cerda, P. A., Crowe-Riddell, J. M., Gonçalves, D. J. P., Larson, D. A., Duda, T. F., & Davis Rabosky, A. R. (2022). Divergent Specialization of Simple Venom Gene Profiles among Rear-Fanged Snake Genera (Helicops and Leptodeira, Dipsadinae, Colubridae). Toxins, 14(7), 489. Scopus10 WoS10 Europe PMC8 |
| 2022 | Folwell, M., Sanders, K., & Crowe-Riddell, J. (2022). The Squamate Clitoris: A Review and Directions for Future Research.. Integrative and comparative biology, 62(3), 559-568. Scopus6 WoS6 Europe PMC4 |
| 2022 | Folwell, M. J., Sanders, K. L., Brennan, P. L. R., & Crowe-Riddell, J. M. (2022). First evidence of hemiclitores in snakes.. Proc Biol Sci, 289(1989), 20221702. Scopus11 WoS10 Europe PMC5 |
| 2021 | Crowe-Riddell, J. M., Jolly, C. J., Goiran, C., & Sanders, K. L. (2021). The sex life aquatic: Sexually dimorphic scale mechanoreceptors and tactile courtship in a sea snake Emydocephalus annulatus (Elapidae: Hydrophiinae). Biological Journal of the Linnean Society, 134(1), 154-164. Scopus7 WoS5 |
| 2021 | García-Cobos, D., Gómez-Sánchez, D. A., Crowe-Riddell, J. M., Sanders, K. L., & Molina, J. (2021). Ecological and sexual roles of scale mechanoreceptors in two species of Neotropical freshwater snake (Dipsadinae: Helicops). Biological Journal of the Linnean Society, 134(4), 958-974. Scopus6 WoS4 |
| 2021 | Callahan, S., Crowe-Riddell, J. M., Nagesan, R. S., Gray, J. A., & Davis Rabosky, A. R. (2021). A guide for optimal iodine staining and high-throughput diceCT scanning in snakes. Ecology and Evolution, 11(17), 11587-11603. Scopus29 WoS27 Europe PMC22 |
| 2021 | Crowe-Riddell, J. M., Dix, S., Pieterman, L., Nankivell, J. H., Ford, M., Ludington, A. J., . . . Allen, L. (2021). From matte banded to glossy black: structures underlying colour change in the caudal lures of southern death adders (Acanthophis antarcticus, Reptilia: Elapidae). Biological Journal of the Linnean Society, 132(3), 666-675. Scopus7 WoS4 |
| 2019 | Crowe-Riddell, J. M., Simões, B. F., Partridge, J. C., Hunt, D. M., Delean, S., Schwerdt, J. G., . . . Sanders, K. L. (2019). Phototactic tails: evolution and molecular basis of a novel sensory trait in sea snakes. Molecular Ecology, 28(8), 1-16. Scopus17 WoS16 Europe PMC13 |
| 2019 | Lillywhite, H. B., Sheehy, C. M., Sandfoss, M. R., Crowe-Riddell, J., & Grech, A. (2019). Drinking by sea snakes from oceanic freshwater lenses at first rainfall ending seasonal drought. PLoS ONE, 14(2), 11 pages. Scopus20 WoS16 Europe PMC8 |
| 2019 | Crowe-Riddell, J. M., Williams, R., Chapuis, L., & Sanders, K. L. (2019). Ultrastructural evidence of a mechanosensory function of scale organs (sensilla) in sea snakes (Hydrophiinae). Royal Society Open Science, 6(4), 16 pages. Scopus26 WoS21 Europe PMC11 |
| 2019 | Crowe-Riddell, J. M., D'Anastasi, B. R., Nankivell, J. H., Rasmussen, A. R., & Sanders, K. L. (2019). First records of sea snakes (Elapidae: Hydrophiinae) diving to the mesopelagic zone (>200 m). Austral Ecology, 44(4), 752-754. Scopus18 WoS17 |
| 2018 | Udyawer, V., Barnes, P., Bonnet, X., Brischoux, F., Crowe-Riddell, J. M., D'Anastasi, B., . . . Voris, H. K. (2018). Future directions in the research and management of marine snakes. Frontiers in Marine Science, Nov 2018(5), 1-16. Scopus33 WoS32 |
| 2016 | Crowe-Riddell, J., Snelling, E., Watson, A., Suh, A., Partridge, J., & Sanders, K. (2016). The evolution of scale sensilla in the transition from land to sea in elapid snakes. Open Biology, 6(6), 160054-1-160054-12. Scopus39 WoS32 Europe PMC19 |
| 2012 | Langmore, N., Feeney, W., Crowe-Riddell, J., Luan, H., Louwrens, K., & Cockburn, A. (2012). Learned recognition of brood parasitic cuckoos in the superb fairy-wren Malurus cyaneus. Behavioral Ecology, 23(4), 798-805. Scopus60 WoS65 |
| Year | Citation |
|---|---|
| 2023 | Crowe-Riddell, J. M., & Lillywhite, H. B. (2023). Sensory systems. In C. Warwick, & P. C. Arena (Eds.), Health and Welfare of Captive Reptiles (pp. 45-91). Springer Link. DOI Scopus13 |
| Year | Citation |
|---|---|
| 2023 | Folwell, M. J., Sanders, K. L., & Crowe-Riddell, J. M. (2023). The squamate clitoris: A review and directions for future research. In INTEGRATIVE AND COMPARATIVE BIOLOGY Vol. 62 (pp. S97-S98). ELECTR NETWORK: OXFORD UNIV PRESS INC. |
| 2020 | Crowe-Riddell, J. M., Pieterman, L., Simoes, B. F., Nankivell, J. H., Ford, M., Ludington, A., . . . Sanders, K. L. (2020). Ontogenetic change in hue and structure of caudal lure reflects dietary shift in Australian death adders (Elapidae). In INTEGRATIVE AND COMPARATIVE BIOLOGY Vol. 60 (pp. E304). Austin, TX: OXFORD UNIV PRESS INC. |
| 2017 | Crowe-Riddell, J. M., Lillywhite, H. B., Partridge, J. C., & Sanders, K. L. (2017). Tail photoreception: investigating a novel sensory system in Australian sea snakes. In INTEGRATIVE AND COMPARATIVE BIOLOGY Vol. 57 (pp. E235). New Orleans, LA: OXFORD UNIV PRESS INC. |
| Year | Citation |
|---|---|
| - | Crowe-Riddell, J., Wagner, A., 0000-0001-6236-0771, S. P. C., & Sanders, K. (n.d.). Dataset for Morphological Evidence for a Directional Flow Mechanoreceptor in Olive‐Headed Sea Snakes (<i>Hydrophis major</i>). DOI |
| - | Crowe-Riddell, J. (n.d.). From matte banded to glossy black: Structures underlying colour change in the caudal lures of southern death adders (Acanthophis antarcticus, Reptilia: Elapidae). DOI |
| - | Crowe-Riddell, J., & Sanders, K. (n.d.). Images of Emydocephalus annulatus scale protuberances. DOI |
| - | Crowe-Riddell, J., & Sanders, K. (n.d.). Histology of Emydocephalus annulatus skin. DOI |
| Year | Citation |
|---|---|
| - | Crowe-Riddell, J., & Sanders, K. (n.d.). Video of courtship behaviour in turtle headed sea snakes. DOI |
| - | Crowe-Riddell, J., & Sanders, K. (n.d.). From matte banded to glossy black: Structures underlying colour change in the caudal lures of southern death adders (Acanthophis antarcticus, Reptilia: Elapidae). DOI |
| Year | Citation |
|---|---|
| 2025 | Wagner, A., Johnson, C., Myoung, H. H., Sanders, K. L., Collin, S. P., & Crowe-Riddell, J. M. (2025). Morphology and distribution of a new scale mechanoreceptor type in olive-headed sea snakes ( <i>Hydrophis major</i> ). DOI |
Fellowships
- Discovery Early Career Researcher Award (DECRA) 2024–2027, Australian Research Council, “Serpent sensory innovation in the evolutionary transition from land to sea” ($423,232; DE240100501)
- Fulbright Postgraduate Scholarship 2016, Australian American Fulbright Commission Sole CI
External funding
2025
- Micro-Computed Tomography Beamline at the Australian Synchrotron 2025, Australia’s Nuclear Science and Technology Organisation (ANSTO), “Bargain with Darkness: Trade-Offs in Morphology and Neurology between Visual and Chemo-sensory Systems in Australian Underground Water Beetles”, (in kind $65,568; M23739) Co-CI
- Minderoo Foundation Exmouth Research Laboratory 2025-2026, Minderoo Foundation, “Assessing chemosensory behaviour and swimming performance to understand bycatch risk in sea snakes” (in kind $108,200) Lead CI
2024
- Hermon Slade Grant 2024-2027, Hermon Slade Foundation, “How do thermosensitive species respond to temperature stress in a warming world? ($87,115) Co-CI
- Our Marine Grants – Round 4 2024-2026, Parks Australia, “Protecting the biological and cultural values of sea snakes under climate change in Dampier Marine Park” ($331,926) Co-CI
2023
- Discovery Project 2023–2026, Australian Research Council, “Plastic brains: Neural adaptations to changing environments in reptiles” ($430,000; DP230101438) Lead CI
- Imaging and Medical Beamline at the Australian Synchrotron 2023, Australia’s Nuclear Science and Technology Organisation (ANSTO), “Nervous and sensory systems of Australian reptiles and fishes: a neuroecological study”, (in kind grant value $65,568) Co-CI
- Our Marine Grants – Round 3 2022–2023, Parks Australia, “Role of Australian Marine Parks in connecting and conserving sea snake populations across northern Australia” ($400,000) Co-CI
- Minderoo Foundation Exmouth Research Laboratory 2021–2022, Minderoo Foundation, “Can eDNA be used to find cryptic populations of critically-endangered sea snakes?” (in kind) Lead CI
- Margaret Middleton Fund for Endangered Vertebrates 2020–2022, Australia Academy of Sciences, “Can eDNA be used to find cryptic populations of critically-endangered sea snakes?” ($18,900) Lead CI
Internal funding
- Elizabeth MacMeikan Trust 2025-2026, University of Adelaide, “Smell and smellilibility: The role of pheromones in reptile sensual behaviour” ($6,638) Sole CI
- Centre for CSEI Education Community of Practice 2025 Seed Funding, University of Adelaide, “Grasping Evolution: 3D Tactile Models of Vertebrate Brains and Skulls” ($4,920) Co-CI
- Strategic equipment grant 2024, Environment Institute at University of Adelaide, high-speed camera for capturing behavioural data ($10,000) Sole CI
- ECRs and MCRs Awards 2021-2022, La Trobe University, “Plastic brains: Neural adaptations to changing environments in reptiles” ($45,255) Sole CI
- R1 Capital Funding for Research Infrastructure 2021, La Trobe University, Gelsight scanner ($30,000) Co-CI
- Strategic and Infrastructure Support Grants 2021, La Trobe University, high powered workstation for processing CT scans ($13,150) Co-CI
Awards
- EMCR Conference Travel Award 2024, School of Biological Sciences ($2700), University of Adelaide
- Associate Dean of Research & Industry Engagement Special Commendation for Exceptional Leaders 2023 ($250), La Trobe University
- Top 15% of applicants for a UNESCO L’Oreal Women in Science Fellowship 2023
- School of Biological Sciences Postgraduate All Round Achievement Award 2019 ($500), University of Adelaide, recognizing academic achievement, and service to the School and scientific community during postgraduate study
- PhD Research Excellence Finalist 2019, South Australian Science Excellence Awards, selected as 1 of 3 finalists, these awards recognize outstanding achievement at a very early stage of an individual’s career
- Dean’s Letter of Commendation for Doctoral Thesis Excellence 2019, University of Adelaide
- Scientific Exchange Program Grant 2019, New Caledonia University (in kind)
- STEM Award Finalist 2017, Channel 9 Young Achiever Awards South Australia, 1 of 3 finalists, these awards encourage, acknowledge and reward the contributions of young people in SA
- South Australian Fresh Scientist 2017, Science in Public, selected as 1 of 10 Fresh Scientists, a national science communication competition that helps early-career researchers share their science stories
- Gregory Swartz Award 2017, Australian American Fulbright Commission ($1000)
- Charlotte Magnum Support Program 2017, Society for Integrative and Comparative Biology (in kind)
- Gans Travel Award 2016, Gans Collections and Charitable Fund ($1000)
- Student Travel Award 2016, Australian Herpetology Society ($200)
- Australian Postgraduate Scholarship 2015, Australian Federal Government (PhD stipend 3 years)
-
Chancellors Letter of Commendation 2009, Australian National University, acknowledging a high-grade point average during undergraduate degree
University of Adelaide
- 2024 - ongoing, Lecturer, Zoology II (ENVBIOL2503), University of Adelaide, 7 lectures on the vertebrate evolution and diversity including a practical class to identify key adaptations that led to mammals and birds. Designing assessment for the practical and lecture test questions.
- 2024 Tutor, Principles & Practice of Research (Advanced) II (SCIENCE2300), University of Adelaide, tutorial on sensory ecology and conservation in 2024.
Past teaching
- 2023 Guest lecturer, Animal Physiology (ZOO2AP), La Trobe University, single lecture on ‘Olfaction in vertebrates’ in 2022 and 2023.
- 2023 Practical demonstrator, Australian Fauna and Ecology (ZOO2FE), La Trobe University, assisting with animal identification practical class including a snake ID task using shed snake skins and dichotomous keys, providing materials (shed skins, preserved specimens) and demonstrating to undergraduate students during three practical classes.
- 2022 Mentor, Work-based Learning Industry Placements (LTU3IND), La Trobe University, academic mentor to a work-placement student, providing training in lab, field and analytical techniques as well as mentorship on career pathways.
- 2020 Co-coordinator and tutor, Project:MORPH! Herpetology (EEB 450), University of Michigan, designing content and quizzes using digital specimens from museum collections for a semester (10 classes), major learning objectives were around form-and-function relationships and interpreting multivariate/multidimensional data in Ecology and Evolution, I was also a mentor for a team of 5 undergraduates guiding them to produce an interactive exhibit for a museum display.
- Co-coordinator and tutor, Graduate Formal Discussion (EEB 800), University of Michigan, designing and delivering for a semester (10 classes) to lead a reading and discussion group for PhD students on the theme of ‘Diversity and Inclusion in Ecology and Evolutionary Biology.
- 2021 Guest lecturer, Exeter Marine Biology, University of Exeter, single online lecture on the sensory evolution of sea snakes and practical advice on pursuing a career in marine biology.
- 2019 Practical demonstrator, Zoology II (ENVBIOL2503), University of Adelaide, assisting with mollusk dissection, introduction to microscopy and scientific illustration and functional morphology of vertebrates, as well as essay marking.
- 2018 Guest lecturer and practical demonstrator, Evolutionary Biology II (ENVBIOL2501), University of Adelaide, single lecture on using phylogenetic comparative methods to identify candidate genes involved in novel sea snake sensory system; practical demonstrator for cladistics and bird diversity in 2015 and 2018.
- 2017 Guest lecturer, Herpetology, University of Florida, single lecture on Sensory Systems in Amphibians and Reptiles.
| Date | Role | Research Topic | Program | Degree Type | Student Load | Student Name |
|---|---|---|---|---|---|---|
| 2026 | Principal Supervisor | Tactile senses in snakes: evolutionary ecology and implications for conservation | Doctor of Philosophy | Doctorate | Full Time | Ms Alizee Wagner |
| 2025 | Principal Supervisor | Anatomical comparison of features used for chemical communication in terrestrial and marine Sauropsida | Doctor of Philosophy | Doctorate | Full Time | Ms Lisa-Marie Kiessling |
| 2025 | Principal Supervisor | Anatomical comparison of features used for chemical communication in terrestrial and marine Sauropsida | Doctor of Philosophy | Doctorate | Full Time | Ms Lisa-Marie Kiessling |
| 2022 | Co-Supervisor | Evolution and conservation genetics of sea snakes | Doctor of Philosophy | Doctorate | Full Time | Miss Amelia Rose Pointon |
| 2022 | Co-Supervisor | Evolution and conservation genetics of sea snakes | Doctor of Philosophy | Doctorate | Full Time | Miss Amelia Rose Pointon |
| 2021 | Principal Supervisor | Reproduction and genital morphology of snakes | Doctor of Philosophy | Doctorate | Full Time | Miss Megan Folwell |
| 2021 | Principal Supervisor | Reproduction and genital morphology of snakes | Doctor of Philosophy | Doctorate | Full Time | Miss Megan Folwell |
| Date | Role | Membership | Country |
|---|---|---|---|
| 2015 - ongoing | - | Sea snake specialist group | - |








Available For Media Comment.