
Dr Danny Wilson
Externally-Funded Research Fellow (C)
School of Biological Sciences
Faculty of Sciences, Engineering and Technology
Eligible to supervise Masters and PhD - email supervisor to discuss availability.
Single cell parasites such as malaria and Toxoplasma remain leading causes of disease in children and during pregnancy, with Australia and our nearest neighbours continuing to be impacted. Malaria alone causes >600,000 deaths per year, with children under five and pregnant women most at risk. The burden of Malaria falls on developing tropical and sub-tropical countries throughout the world, with the health and prosperity of Australia's nearest neighbours and major trading partners still heavily affected by these mosquito borne parasites. Up to a third of the worlds population is infected with food and water borne Toxoplasma parasites that can cause severe disease during pregnancy and for immunocompromised people, with the long-term impacts of infection not fully understood.
Our research applies multi-disciplinary approaches to better understand the unique biology of these parasites. We use this information to prioritise and develop vaccines that protect against infection and for the identification of new drug treatment strategies to cure people infected with these widespread disease causing parasites.
- My Research
- Career
- Publications
- Grants and Funding
- Teaching
- Supervision
- Professional Activities
- Contact
Plasmodium spp. (malaria) parasites remain one of the world’s most deadly infectious agents. The related parasite Toxoplasma gondii infects a third of the world’s population and likely contributes to chronic disease and causes severe and fatal infections during pregnancy. Through national/international collaborations and supported by competitive and industry funding, we are applying in vitro and in vivo approaches to identify and develop new drug and vaccines against malaria and Toxoplasma. We also explore their unique, and potentially druggable, adaptations that help them survive.
Half of the world’s population is at risk of infection with mosquito-borne malaria parasites of the genus Plasmodium. These complex eukaryotic parasites that live inside human cells cause widespread sickness and death throughout tropical and sub-tropical regions of the world including countries neighbouring Australia. The majority of the >600,000 malaria related deaths that occur each year are caused by the parasite Plasmodium falciparum in children under 5 years in Africa, Asia and South America. Unfortunately, resistance has developed to our most effective anti-malarial drugs, resulting in poorer treatment outcomes for clinical cases of this deadly parasite. In the case of T. gondii, which infects wildlife, livestock and up to a third of the worlds human population, we are reliant on old drugs of questionable efficacy. There is an urgent need to identify novel drugs to partner with current first-line treatments to fill these treatment gaps. In addition, development of effective vaccines would reduce the burden of disease and greatly facilitate efforts to eradicate malaria and control T. gondii infection. Unfortunately, a highly effective vaccine to protect against malaria or T. gondii has not been developed to date and efforts to identify the best vaccine targets are ongoing.
 Antimalarials that inhibit invasion and parasite growth
Antimalarials that inhibit invasion and parasite growth
My laboratory seeks to identify and characterise novel parasite proteins for their suitability as both drug and vaccine targets. We look at drugs that block invasion of the human red blood cell by the small invasive merozoite stage of the malaria parasites lifecycle as a therapeutic target. We also work with small molecule and natural products that inhibit malaria parasite or T. gondii growth at nanomolar concentrations as potential new tools to investigate biology and for clinical development.
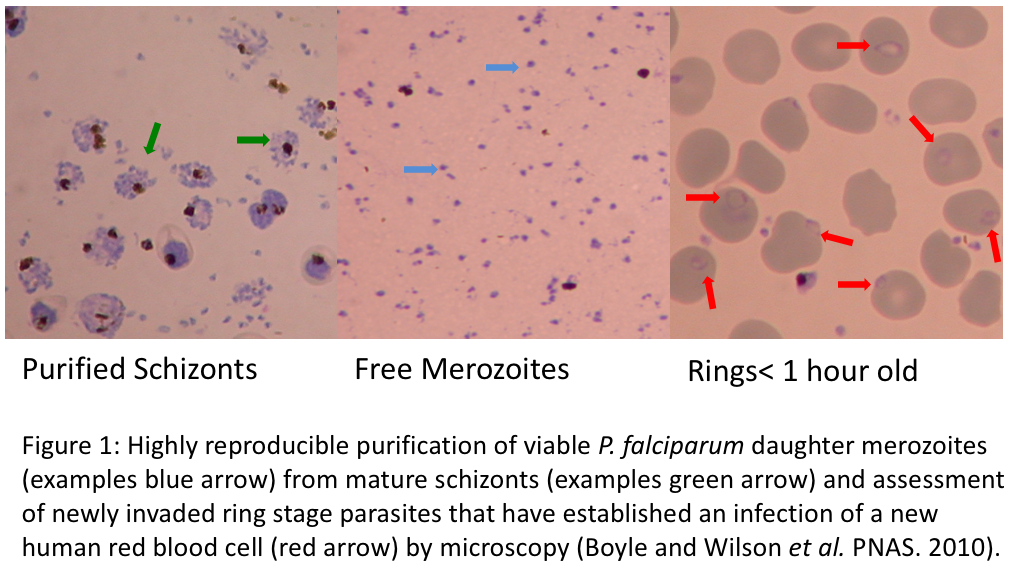
By applying state of the art methods, we aim to develop anti-malarial and anti-Toxoplasma drugs that can be used to partner or replace current frontline treatments. Using a breakthrough P. falciparum merozoite purification method (PNAS, 2010; Figure 1) and a highly reproducible flow cytometry approach (Antimicrobial Agents & Chemoth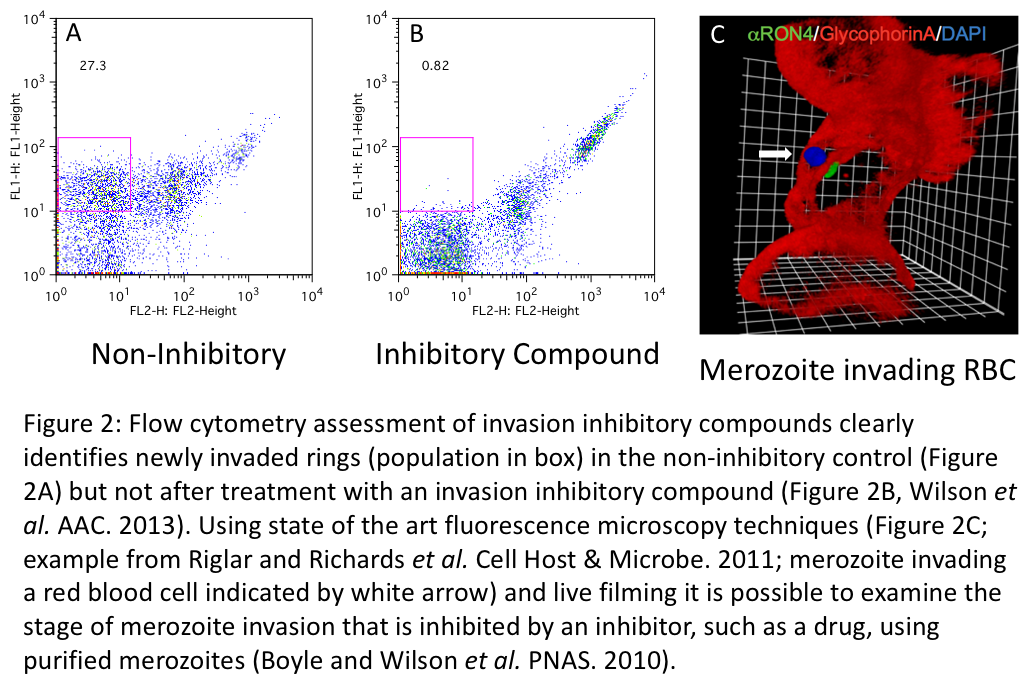 erapy, 2013; Figure 2 A & B), we have screened traditional antimalarial therapies, protease inhibitors, kinase inhibitors, a range of antibiotics and identified several compounds and there analogues that rapidly inhibit merozoite invasion (BMC Biology, 2015). Using standard in vitro assays, we have also screened several thousand small molecules and natural products for their broad spectrum activity against malaria parasites and Toxoplasma gondii. To understand how drug-like compounds kill the parasites, we apply mutation analysis of gene targets, phenotypic screens of related Apicomplexan parasites, modifications and screens of analogues, fluorescence microscopy localisation and proteomic identification of drug binding targets. Importantly, many of the invasion inhibitory compounds identified in our research also kill at other stages of the parasites complex lifecycle, increasing their utility as broad acting antimalarials. Development of both invasion inhibitory and broad acting compounds and identification of the mechanisms by which they inhibit invasion is the subject of ongoing research in the laboratory.
erapy, 2013; Figure 2 A & B), we have screened traditional antimalarial therapies, protease inhibitors, kinase inhibitors, a range of antibiotics and identified several compounds and there analogues that rapidly inhibit merozoite invasion (BMC Biology, 2015). Using standard in vitro assays, we have also screened several thousand small molecules and natural products for their broad spectrum activity against malaria parasites and Toxoplasma gondii. To understand how drug-like compounds kill the parasites, we apply mutation analysis of gene targets, phenotypic screens of related Apicomplexan parasites, modifications and screens of analogues, fluorescence microscopy localisation and proteomic identification of drug binding targets. Importantly, many of the invasion inhibitory compounds identified in our research also kill at other stages of the parasites complex lifecycle, increasing their utility as broad acting antimalarials. Development of both invasion inhibitory and broad acting compounds and identification of the mechanisms by which they inhibit invasion is the subject of ongoing research in the laboratory.
 Functional characterisation of essential malaria proteins and vaccine targets
Functional characterisation of essential malaria proteins and vaccine targets
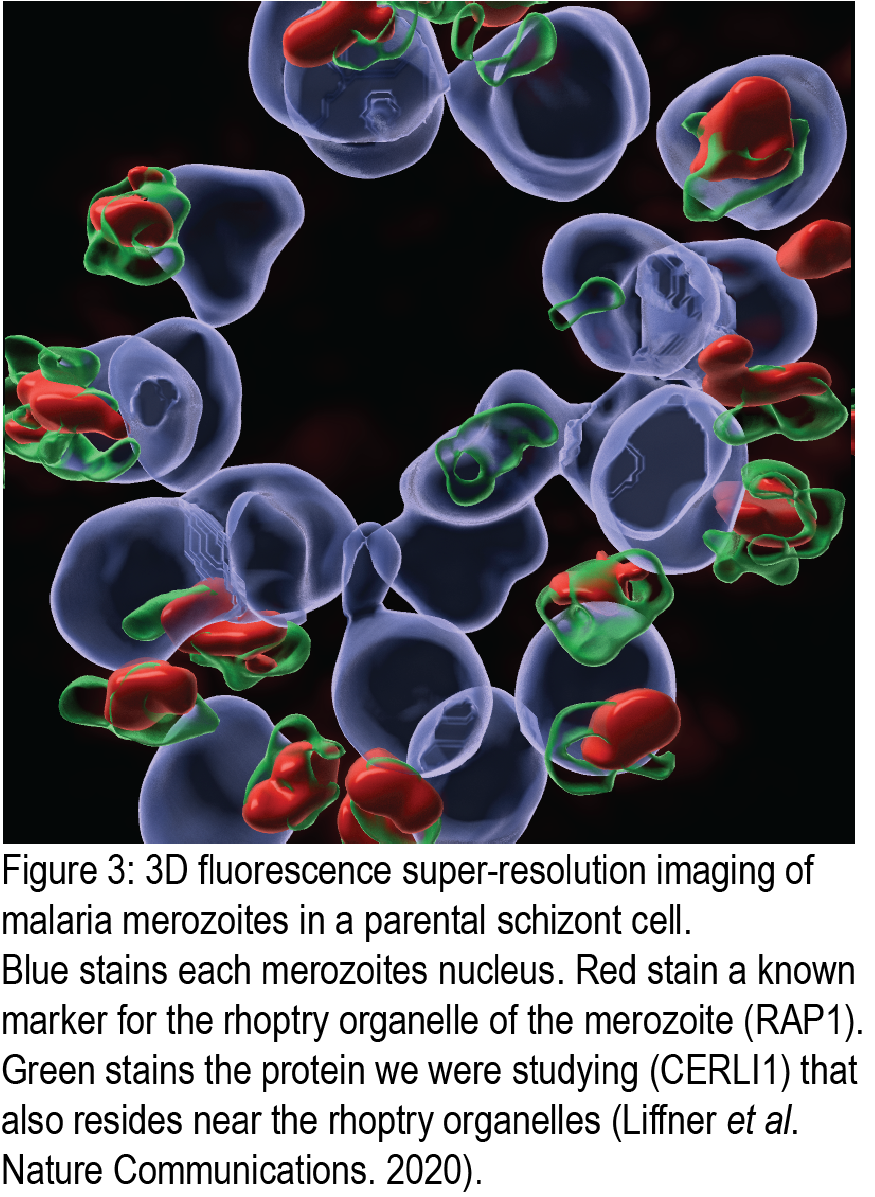
The function of nearly 50% of the genes in P. falciparum parasites is poorly described or not known altogether. Malaria proteins involved in invasion are no exception with a large number of potential vaccine and drug targets having no known function. We apply gene-editing techniques to examine the potential role of some of these proteins in invasion and parasite growth. Using fluorescence microscopy (Figure 3), flow cytometry, genetic manipulation and proteomic techniques, we are characterising the localisation, interactions and functions of merozoite antigens with no known function to test their suitability as therapeutic targets.
Postgraduate and undergraduate opportunities
Honours, summer-student, masters and PhD projects are available to explore the biology of the malaria parasite and to develop new therapies against this deadly infectious organism. We are a small, supportive, research team that is applying new technologies to discover new ways of ending the burden of malaria disease. If you would like to find out more about our current research opportunities and whether our research is right for you, please contact Dr. Danny Wilson (danny.wilson@adelaide.edu.au).
-
Appointments
Date Position Institution name 2017 - 2019 DVCR 3 Year Mid-career Beacon Fellowship University of Adelaide, Adelaide 2015 - ongoing Laboratory Head University of Adelaide 2014 - ongoing Burnet Institute Research Fellow (Honorary) Burnet Institute, Melbourne 2014 - 2015 Senior Research Fellow (with Prof. James Paton) University of Adelaide 2012 - 2015 Peter Doherty, Early Career Fellowship National Health and Medical Research Council, Canberra 2011 - 2013 Senior Research Fellow (with Prof. Alan Cowman) Walter and Eliza Hall Institute of Medical Research, Melbourne 2009 - 2011 Research Officer (with Prof. James Beeson) Walter and Eliza Hall Institute of Medical Research, Melbourne -
Awards and Achievements
Date Type Title Institution Name Country Amount 2020 Award Faculty of Sciences Mid-Career Researcher Excellence Award The University of Adelaide Australia - 2020 Fellowship Mid-career Fellowship (2020-2022) The Hospital Research Foundation Australia - 2019 Award Young Tall Poppy SA Award AIPS Australia - 2017 Fellowship DVCR, Beacon Fellowship (2017-2019) The University of Adelaide Australia - 2015 Award Faculty of Sciences Early Career Researcher Travel Award The University of Adelaide Australia - 2013 Award CASS Foundation travel Grant CASS Foundation Australia - 2012 Award Post Doctoral Association travel award Walter & Eliza Hall Institute Australia - 2012 Fellowship Peter Doherty Early Career Fellowship NHMRC Australia - -
Education
Date Institution name Country Title 2004 - 2009 University of Melbourne, Melbourne Australia PhD -
Research Interests
-
Journals
-
Conference Papers
Year Citation 2010 Boyle, M. J., Wilson, D. W., Richards, J. S., Riglar, D. T., Tetteh, K. K., Conway, D. J., . . . Beeson, J. G. (2010). ISOLATION OF VIABLE <i>PLASMODIUM FALCIPARUM</i> MEROZOITES TO DEFINE ERYTHROCYTE INVASION EVENTS AND ADVANCE VACCINE AND DRUG DEVELOPMENT. In AMERICAN JOURNAL OF TROPICAL MEDICINE AND HYGIENE Vol. 83 (pp. 127-128). Atlanta, GA: AMER SOC TROP MED & HYGIENE. -
Preprint
Year Citation 2023 Foley, M., Angage, D., Anders, R., Chmielewski, J., Maddumage, J., Hesping, E., . . . Boddey, J. (2023). A broadly cross-reactive i-body to AMA1 potently inhibits blood and liver stages of Plasmodium parasites.
2020 Liffner, B., Balbin, J. M., Shami, G., Strauss, J., Frölich, S., Heinemann, G., . . . Wilson, D. (2020). <i>Pfcerli2</i>, a duplicated gene in the malaria parasite<i>Plasmodium falciparum</i>essential for invasion of erythrocytes as revealed by phylogenetic and cell biological analysis.
2023 - DAAD/Universities Australia-Germany Joint Research Co-operation Scheme (2024-2025). Lead-CI: Dr. Danny Wilson.
2023 - South Australian Sheep Industry Fund, Project Grant. CIB: Dr Danny Wilson.
2023 - Davies Livestock Research Centre Seed Grant. CIA Dr Danny Wilson.
2022 - NHMRC Ideas Grant: APP2020822 (2023-26). CIA Dr. Danny Wilson.
2022 - ARC-ITTC: IC220100050 (2023-28). ARC Training Centre for Environmental and Agricultural Solutions to Antimicrobial Resistance (ARC CEA-StAR). Co-CIA Dr Danny Wilson.
2022 - Humboldt Foundation, Mid-Career Fellowship. CIA: Dr Danny Wilson.
2022 - The University of Adelaide, DVCR, 3-Year Future Making Fellowship. CIA: Dr. Danny Wilson.
2021 - Hospital Research Foundation (2021-24), Collaborative Grant. CIA: Dr. Danny Wilson
2020 - NHMRC Ideas Grant: APP2003712 (2021-23). CIA Dr. Danny Wilson.
2020 - ARC LIEF (LE210100155). Advancing 4D fluorescence microscopy within Australia. CID and Management Team: Dr. Danny Wilson
2019 - Hospital Research Foundation, 3-Year Mid-career Fellowship. CIA: Dr. Danny Wilson
2019 - South Australian Sheep Industry Fund, Project Grant. CIB: Dr Danny Wilson
2019 - DAAD/Universities Australia-Germany Joint Research Co-operation Scheme (2020-2021). Lead-CI: Dr. Danny Wilson.
2017 - NHMRC Project Grant: APP1143974 (2018-21). CIA Dr. Danny Wilson.
2017 - The University of Adelaide, Cross-Faculty Equipment Call. Oxford Nano-imager NIM 4 Microscope System. Lead CI: Dr. Danny Wilson
2017 - Channel 7 Children's Research Foundation Project Grant. CIA: Dr. Danny Wilson
2017 - DAAD/Universities Australia-Germany Joint Research Co-operation Scheme (2018-2019). Lead-CI: Dr. Danny Wilson.
2016 - The University of Adelaide, DVCR, 3-Year Beacon Fellowship. CIA: Dr. Danny Wilson.
2016 - The University of Adelaide, DVCR, Research Infrastructure Scheme. Advanced confocal microscope. CIA: Dr. Danny Wilson.
2016 - The University of Adelaide, DVCR, Interdisciplinary Research Fund. Imaris microscopy analysis software. CIA: Dr. Danny Wilson.
2015 - The University of Adelaide, DVCR, Interdisciplinary Research Fund. Metalloproteomics HPLC. CID: Dr. Danny Wilson.
2013 - NHMRC Project Grant: APP1057960. CIC Dr. Danny Wilson.
2011- NHMRC Peter Doherty Early Career Fellowship APP1035715. CIA Dr. Danny Wilson.
2004- NHMRC Dora Lush PhD Scholarship 2004-2008: APP305553. CIA: Dr. Danny Wilson.
- 3rd Year: Infection and Immunity A, 2 lectures, Semester 1.
- 2nd Year: Biomedical Science, 2 lectures, Semester 2.
- 2nd Year: BSc Advance Mentor, Semester 1.
- 1st Year: SGDE for human perspectives, Semester 2.
-
Current Higher Degree by Research Supervision (University of Adelaide)
Date Role Research Topic Program Degree Type Student Load Student Name 2024 Principal Supervisor Characterisation of Cross-Species Function for Malaria Merozoite Proteins 4 and 5 Master of Philosophy Master Full Time Mr Jarryd Blake Tiu 2023 Principal Supervisor Characterising key machinery that orchestrates malaria parasite host cell entry Doctor of Philosophy Doctorate Full Time Mr Shamit Singla 2023 Principal Supervisor Functional characterisation of novel proteins in essential malaria protein complexes Master of Philosophy Master Full Time Miss Meghan Emma Zadow 2023 Principal Supervisor Characterisation of cytosolically exposed rhoptry leaflet interacting protein interacting partners that are essential for malaria entry into host cells Master of Philosophy Master Full Time Miss Mia Andrews 2023 Principal Supervisor Characterisation of novel drugs with therapeutic potential against the pathogenic parasites malaria and Toxoplasma gondii Master of Philosophy Master Full Time Miss Emma Yuxin Mao 2023 Principal Supervisor Identifying Plasmodium falciparum and P. vivax surface antigens that are targets of functional antibody responses Doctor of Philosophy Doctorate Full Time Ms Kaitlin Grace Pekin 2023 Principal Supervisor Characterisation of malaria merozoite surface protein function and vaccine potential Doctor of Philosophy Doctorate Full Time Miss Keng Heng Lai 2021 Co-Supervisor Development of methods to control toxoplasmosis and associated apicomplexan parasites in livestock Doctor of Philosophy Doctorate Full Time Mr Quinn Phillip Mackie -
Past Higher Degree by Research Supervision (University of Adelaide)
Date Role Research Topic Program Degree Type Student Load Student Name 2022 - 2024 Co-Supervisor The Biogenesis and Homeostasis of the Shigella flexneri Cell Envelope Doctor of Philosophy Doctorate Full Time Miss Alice Ascari 2021 - 2023 Principal Supervisor Insights into the Biological Constraints on Malaria Red Blood Cell
Invasion and the Implications for Vaccine DevelopmentDoctor of Philosophy Doctorate Full Time Miss Jill Chmielewski 2019 - 2022 Principal Supervisor Functional characterisation of putative Plasmodium falciparum invasion ligands and their homologues Doctor of Philosophy Doctorate Full Time Mr Juan Miguel Balbin 2018 - 2021 Principal Supervisor Characterisation of the Function and Vaccine Potential of Surface Exposed Antigens Required for Malaria Parasite Invasion of Human Host Cells Doctor of Philosophy Doctorate Full Time Ms Isabelle Grace Henshall 2017 - 2020 Principal Supervisor Functional Characterisation Of Novel Plasmodium falciparum Proteins And Their Role In Erythrocyte Invasion. Doctor of Philosophy Doctorate Full Time Mr Benjamin Scott Liffner 2016 - 2020 Principal Supervisor Investigation of azithromycin analogues and proteasome-like inhibitors as quick-killing antimalarials Doctor of Philosophy Doctorate Full Time Miss Amy Lee Burns
-
Board Memberships
Date Role Board name Institution name Country 2016 - 2019 Representative Australian Society for Parasitology, SA Representative - Australia -
Committee Memberships
Date Role Committee Institution Country 2017 - ongoing Secretary Molecular Life Sciences Microscopy Committee University of Adelaide Australia 2016 - 2018 Representative Australian Society for Parasitology, Education Committee Australian Society for Parasitology Australia 2013 - 2013 Treasurer Malaria in Melbourne Organising Committee - Australia -
Memberships
Date Role Membership Country 2017 - ongoing Member Light Microscopy Australia Australia 2014 - ongoing Member Adelaide Protein Group Australia 2014 - ongoing Member Australian Society for Biochemistry and Molecular Biology Australia 2013 - ongoing Representative Australian Society for Parasitology Australia
Connect With Me
External Profiles




